Potts of Leeds and the Settle to Carlisle Railway
Many years ago I bought an old railway clock from a well-known auction website. The clock came from Reddish railway station and consisted of a large glass-fronted dial and a small A-frame mechanism which originally drove it. The dial was marked “Potts & Sons Leeds”. This clock dial and mechanism must be over 100 years old.

The firm of W Potts & Sons was a Leeds based manufacturer of church clocks and other large mechanisms. Potts installed very many clocks of all sizes in railway stations. These were found throughout the Midland Railway. Often a station would have large clock for the platform, and several smaller wall clocks in the offices and booking office. Potts supplied all of these to the Midland Railway.
The fabulous Settle to Carlisle route was orginally built by the Midland Railway, and Potts clocks were installed at every station. The Settle Carlisle Railway Trust website shows a photograph of the Potts dial to be found at Horton in Ribblesdale station, and says
Station Clocks
A small but essential feature on every station
Before the railways came in the 19th century, time was not the same in every place. This made it difficult to run the railways so standard ‘railway time’ was introduced. Every station had a clock made by William Potts & Sons of Leeds. Over the years the clocks fell into disrepair and only the one at Appleby survived. The Trust has restored the clock at Kirkby Stephen and installed five new clocks at Horton, Ribblehead, Dent, Langwathby and Lazonby.
The clock dials now on the platforms at those stations listed were supplied new in 2005 as replicas manufactured by the firm Smith (of Derby), originally a competitor firm which absorbed Potts’ business in the 1930s.
I contacted the Settle & Carlisle Railway Trust and asked them if they would like to acquire as a donation the original Reddish station dial (and its original mechanism). They were very keen to do so, and so we took a trip to Ribblehead Station, and delivered the dial and mechanism to their care.
We are very pleased to be able to support the work of the Settle & Carlisle Railway Trust in recreating the orginal appearances of the historic stations, complete with Potts clocks.



Revision to GAFTA sampling rules
Since I started in this business in the 1990s, much of my work has been with grains and animal feedstuffs. Huge quantities of grain and feeds are traded by sea, and it is no exaggeration to say that this trade is necessary to feed the world. It is a widely quoted statistic that 80% of the world trade in grain and animal feeds is by means of GAFTA contracts.

GAFTA is an acronym for the Grain and Feed Trade Association. I’m pleased to be able to say that Sheard Scientific is a member of GAFTA.
Trade based on GAFTA contracts usually adopts the concept that the definitive and “final” check on the quality of the goods being traded occurs at the time of loading. This enables a degree of certainty to those participating in the trade, but the concept does require reliable sampling and analysis. That sampling is required to be carried out according to GAFTA 124 sampling rules.
I may write in more detail about sampling as a general topic in a later post (time allowing!), but suffice it to say here that representative samples by definition are to represent the average properties of the parcel of cargo being sampled. Since those samples have to be much smaller in size than the cargo they are drawn from, they have to be drawn in a manner which gives equal statistical chance for any given particle in the cargo being sampled to be in the working/drawn sample.

The purpose of GAFTA 124 is to set a standard for how that is to be carried out, how the integrity of the samples should be preserved, and what should be done with them. Whilst the official scope of GAFTA 124 is in reference to the GAFTA sales and purchase contracts, to ensure quality parameters are properly and reliably assessed when a cargo is traded, it is in my experience so commonplace as to be universal that they are used as a benchmark “gold standard” for sampling of cargoes where there is a dispute – in circumstances where there has been transit damage, for instance. The GAFTA sampling rules don’t necessarily suit all such circumstances, but they are always where surveyors and experts start when considering how to sample average parameters of bulk grain or feeds.
Bearing that in mind, a change to the GAFTA 124 rules is potentially big news, and so I read with interest when I received notification of the changes. There have been substantial changes to the GAFTA 124 rules over the years I have been working in this industry – when I first started, in all cases sublots were 500MT in size – meaning dozens of sublots for a moderate sized vessel. That has long-since been changed so that the selection of sublot sizing scales with the overall consignment quantity.
One change being introduced in the new version of the rules is that there is greater clarity regarding the numbers of individual bags from which samples must be taken when bagged goods are being sampled.
Paragraph 9.1.8 now contains the following text, seemingly to ensure that all are made aware of the status of “certificate final” provisions and that sets of samples reserved for arbitration are not to be used to overturn compliant load port certification.
Arbitration samples drawn for goods traded on contracts stating ‘Quality (Certificate) Final’ at loading or at discharge cannot be used to challenge the quality specifications which are included in the ‘Quality (Certificate) Final’ certificate, unless otherwise determined by arbitrators or Board of Appeal.
But to my eyes, the biggest change is in the selection of laboratories. Again, back when I started this work, in the case of any disputes or disagreements, specific laboratories were to be used as “referee” laboratories. In recent years there have been four designated as referee laboratories and their identities were written into GAFTA 124. The four reference laboratories were undeniably “household names” to all in the grain trade, and will no doubt continue to be used as reliable grain testing facilities. However, it is now the case that any GAFTA-approved laboratory can serve this purpose as all approved labs are considered equivalent.

What makes a cargo scientist?
It has been a while since I wrote something for this blog and website. In this post I will talk about my work in general terms.
You may have seen the phrase “cargo scientist” being used to describe people who work in my line of business, but where does that phrase come from and what does it mean? “Cargo scientist” is a phrase coined in recent years, possibly because there hasn’t really been a better way of describing the various aspects of the role.
In the olden days, much of the work carried out by my predecessors was in relation to grain and animal feeds – crops. There was a tendency for those who provided expert evidence in this field to be described by the legal profession who instructed them as “agronomists”. The dictionary definition of an agronomist is “an expert in the science of soil management and crop production”. That then is a fairly clear expression of something I don’t ever do! All of my work involving grains and animal feedstuffs relates to the post-harvest handling and storage of those agricultural materials. Most of it involves carriage at sea, which has some similarities but many differences to land-based storage facilities such as silos. That’s just the agricultural products.
I also provide advice on other types of commodity; coal and wood pellets, ores and minerals, fertilisers, chilled and frozen produce, edible oils and fats, chemicals, and so on; most things that can be put on a ship and carried whether in bulk or packaged into bags or drums. Then there are the more abstract topics which result in problems and disputes – cargo liquefaction, ventilation as its own topic, fumigation, sampling and analysis.
To be a good cargo scientist you need a number of things.
Firstly, you need a strong grounding in the natural sciences. Mostly the knowedge and concepts required are “A” level or early years University level in complexity, but any one case may involve knowledge of physics, chemistry and biology, applied to the various aspects in a case. Thus, as an example, deterioration of soya beans may involve microbiological growth. That requires an understanding of the lifecycle and conditions for growth of yeasts and moulds. Once parts of the stow heat, there may be a temperature gradient or condensation, considerations about which require an understanding of physics. Published data on temperature and moisture for soya beans may need to be converted from one set of units or basis to another (some maths required!). Evaluating the loss to the oil in the beans requires an understanding of the chemistry of oil hydrolysis. And so on.
I’m fortunate that my undergraduate degree course involved studying (initially) three sciences rather than specialising in one subject from the first year. This gives me a broad grounding in the fundamentals of science. I have since starting this work studied for further qualifications, and am still doing so.
Secondly, you need a wide knowledge of the types of cargo carried at sea and an understanding of what the relevant issues or problems with each are likely to be. This knowledge and experience tends to increase with time spent in the industry.
Thirdly, you need to have an analytical mind and the ability to approach unknown problems with a flexible but systematic approach. Even though I have been in this business for most of my working life, and have over 25 years’ experience in cargoes, there are still some commodities or specific tests/parameters that I experience for the first time. Being a cargo scientist means that I know how to approach unknowns and establish a way of tackling the problem and gaining the necessary background knowledge from published data or other sources.
All of these aspects of the work have one thing in common – the use of a scientific approach to explain, diagnose or predict the behaviour of cargoes on ships or in storage. Occasionally advanced or complex science/mathematics is required, but in most instances that isn’t the case. That is one reason why most cargo scientists have Ph.D degrees. It isn’t because the topic studied for the doctorate is necessarily of value to the cargo science job – it rarely is. Studying for a Ph.D involves learning how to carry out research into unfamiliar topics, and how to access and reference scientific literature – essential skills for working as a cargo scientist.

So what do I actually do? Over the years I have worked in this industry, I have dealt with many types of cargo problem in the field as set out above. I’ve climbed ladders, shovelled mouldy grain, taken a very large number of photographs of cargoes, measured temperature on hundreds if not thousands of occasions (why temperature? – that will be the subject of a later post), watched grain flow or not flow during discharge, seen fertiliser on ships and under test in laboratories, sampled cargoes on many occasions, dealt with fires first-hand, seen liquefied bulk cargoes, tested bulk cargoes for transportable moisture limit, witnessed analysis of many different kinds, and have had numerous heated discussions with cargo receivers and surveyors!
The other part of the role of a cargo scientist is reviewing documentary evidence about a case, considering the technical aspects of it, writing expert reports, reviewing opponent expert reports and providing commentary on them, holding expert meetings, and ultimately, providing an expert witness service in Court or Arbitration proceedings (on paper or live in hearings). I have worked on a very large quantity of all aspects of these kinds of cases.
Times have changed since I first starting work in this field. We had one mobile phone for the company, and that didn’t work in most overseas countries. Many times I flew somewhere to discover on arrival that the problem wasn’t quite what the client thought it was. Now, in contrast, there are many excellent surveyors in ports worldwide (hello to any of them reading this!); they all have mobile phones with good cameras. Rather than having to wait weeks or months for a report, clients receive regular updates with photographs or even video footage. From the UK I am able to direct matters remotely, holding video calls when I need to see things myself. The sequence of lockdowns and travel restrictions during the height of the COVID pandemic showed the world that it was possible to investigate and resolve many matters without being able to fly experts around the world. For many cases now, the huge costs of someone travelling out to a vessel are simply not required.
I knew when setting up Sheard Scientific as a new entity that there was plenty of room in the market for a new organisation offering cargo scientist expertise. That has been the case for as long as I have worked in this field – many years ago the field was dominated by Dr RF Milton & Partners, later Jarrett Kirman & Willems, but there were a substantial number of individuals specialising in one type of cargo or another to service the clients’ requirements for independent organisations to instruct. There are fewer such independent indviduals operating today – the expertise has become concentrated in a very small number of firms worldwide, some of which are owned by larger entities.
There is, in my view, no substitute for genuinely independent scientifically-based expertise coupled with vast experience in the field and in providing expert evidence. That is what we offer.
Season’s Greetings
Season’s Greetings from Sheard Scientific to all our friends and clients. Wishing a very Merry Christmas and Happy New Year to all.

Direct Reduced Iron – one of the most unpleasant cargoes out there
It really is. But why?
This article will, I hope, give you some idea why. What it won’t do is catalogue the different types of DRI you might encounter and what the requirements are to handle or carry them safely. That might be a future article, but this one is meant to explain the science underlying DRI and what gives rise to its hazards.
Iron is a very familiar substance – we make all sorts of things out of it or things based on it. Steel structures are found in buildings, cars are made from steel, we have iron railings and gates, and there are many many more examples. There’s a property in common with many of these man made objects, and that is that they rust if you don’t prevent it. Rusting is a very familiar example of an oxidation reaction. Iron plus oxygen becomes iron oxide, one type of which is the familiar red/brown rust we see on iron railings and the steelwork of older cars.

There is lots of iron around in the earth’s crust – that is because the familiar isotopes of iron are, in nuclear physics terms, the most stable substances known. Nuclear stability arises from the lack of tendency to undergo nuclear fission or nuclear fusion. Those two processes release energy from the nucleus when that nucleus splits (fission) or merges with another (fusion). Splitting a nucleus of iron will always cost energy, similarly trying to fuse iron with other nuclei will also absorb energy. Thus iron nuclei are highly stable, and form a substantial proportion of the end-products of the explosions known as supernovae and in other stellar processes. As these are where the elements making up the earth (including those in you and me!) are formed, the presence of large amounts of iron (and also nickel) in the earth’s crust and core is unsurprising. But I digress.
Iron is a moderately reactive metal and is always found in nature combined with other substances as ores. Iron ore might contain iron oxides, iron sulphides, or other forms.
Production of metallic iron from naturally occurring iron ores was first carried out by mankind over 3000 years ago. It was such an important development that the term “iron age” describes the period when iron became available for use. Early furnaces could not reach the melting point of iron and produced a form of the metal known as a “bloom”. Later furnaces used forced air draughts to reach higher temperatures and are known as blast furnaces. These produce molten iron, known as “pig” iron which can then be used in steelmaking.
Ironically (geddit?) DRI has more in common with obsolete bloom iron than more modern blast furnace forms. Consider an ore which is mostly iron oxide. Chemically, this is an example of an ionic material. The iron atoms are present as positively charged iron ions, and the oxygen atoms are present as negatively charged ions. In a piece of solid iron ore, between each pair of adjacent iron atoms is found an oxygen atom, and vice versa.
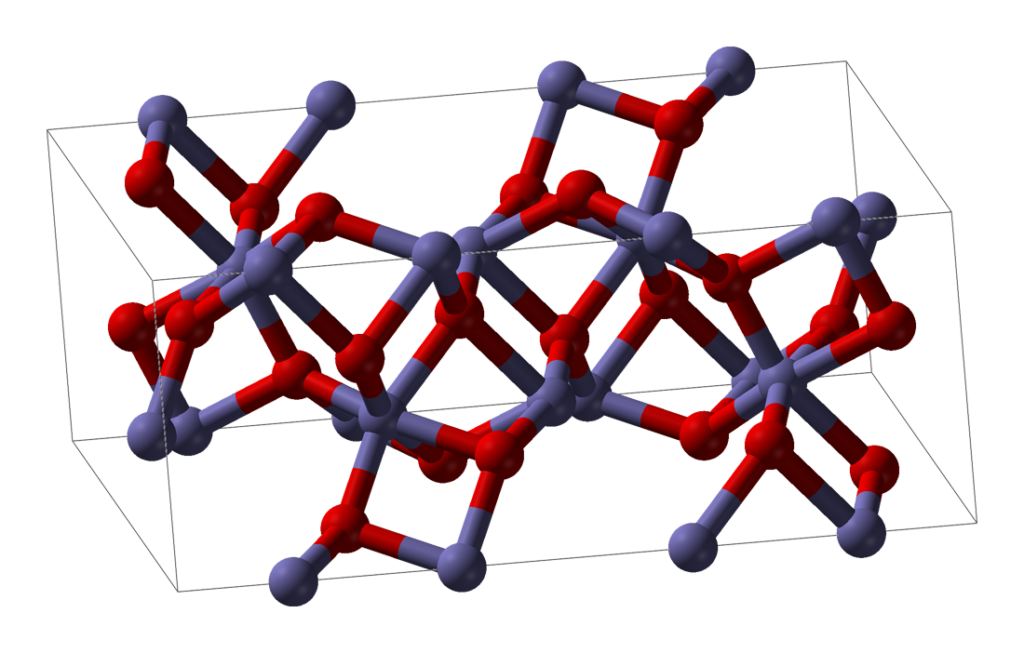
Direct reduction involves the removal of the oxygens without melting the feedstock. Typically the feedstock is pelletised iron ore, each pellet being roughly 1cm in diameter. When the oxygen is removed from the pellet without melting the pellet, the shape and size is retained but the locations which used to have oxygen atoms in them now have gaps. These tiny gaps are all interconnected and the resulting pellet of DRI is extremely porous. It has a huge surface area yet weighs less than the pellet it was made from. Once reduced, it looks very much like a malteser (without the chocolate coating).

I’m going to refer to DRI made by the MIDREX process, which is made in a shaft furnace. The reducing agent (i.e. the material used to remove the oxygen) is hydrogen and carbon monoxide made from natural gas. Natural gas is also used to heat the furnace, but because the ore is not melted, less heat is required than other furnace types, meaning that the DRI process is relatively energy-efficient. The need for natural gas to heat the furnace and provide the reducing gas means that DRI plants are usually found in locations where gas is plentiful and relatively inexpensive.
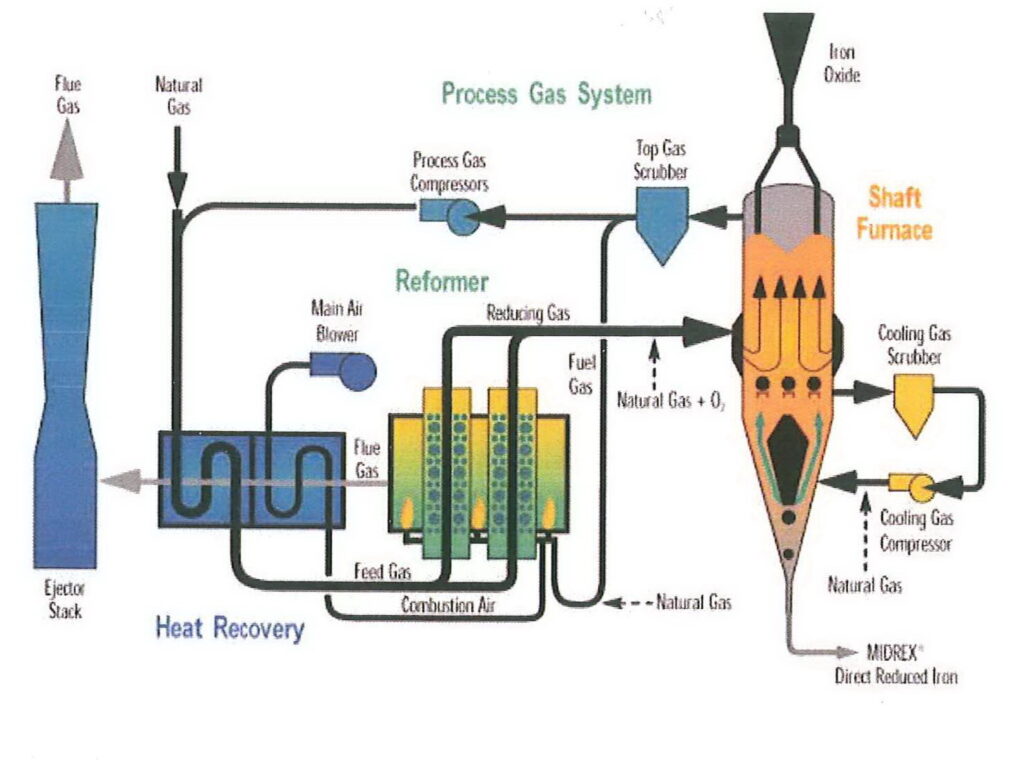
A parameter often quoted in the context of DRI is % metallisation. This is the percentage of the iron in the pellet which is present as free metallic iron rather than oxides. With modern DRI facilities, metallisation will be 95% or so. This iron can be used directly in steelmaking.
Because DRI production tends to take place where the natural gas is, it is often then carried by sea to a steel mill. That is unfortunately where some of the problems arise.
All of the problems associated with DRI come about because of the physical form that iron is in. A lump of iron can rust at its surface. That rusting usually involves water, and rusting by sea water is more rapid than with fresh water. Rusting to a cargo of DRI, especially if wetted by sea water ingress, can damage the cargo by allowing parts of it to revert to iron oxide. Loss of cargo value is undoubtedly something a shipowner will want to avoid, but there are much more immediate problems a vessel is likely to face if DRI becomes damaged on board.

Rusting is an oxidation reaction, and like all oxidative reactions, involves the release of heat. Quite a lot of heat in chemical terms, but we don’t notice a rusty car or iron railing getting hot as it rusts. Why not?
The answer is that the rusting only happens at the exposed surface of the railings or car structure, and does so slowly enough that any heat generated is immediately lost to the atmosphere. There’s never a temperature rise.
In contrast, a pellet of DRI has a huge surface area – some estimates suggest that a pellet of DRI has a surface area 10000 greater than a lump of solid iron. That means that when the DRI is freshly produced it has 10000 times the capability of reacting with oxygen and rusting. That’s one reason that DRI behaves differently to a lump or a gate made of iron.
The other hugely significant property of DRI is that, like most bulk cargoes, it is a poor conductor of heat. That might be a little surprising to hear as there is nothing there other than iron, and metals conduct heat, don’t they?
A block of iron does indeed conduct heat. A bulk made up of spheres made out of iron does not conduct very well because the contact area between adjacent spheres is small and thermal contact is poor. A bulk made up of DRI, in which much of the surface area is space rather than metal conducts heat very badly indeed.
That means that where (for example), sea water enters a bulk stow of DRI, the first thing which happens is that the wetted iron rusts. Fresh water will cause rusting but sea water makes it happen much more rapidly. The heat released by the rusting reaction, which for most of the forms of iron we encounter everyday is lost immediately, does not get conducted away and makes the temperature rise locally where the DRI was wetted.
As the temperature of the iron at those points within the stow rises, it may reach a high enough temperature that the iron can react directly with the oxygen in the hold (if there is any!). This isn’t rusting any more but is direct oxidation. The higher the temperature gets, the more rapidly the reaction goes.
If there is a ready supply of fresh air/oxygen, a DRI cargo can heat to several hundred degrees Celsius and will keep going. Whilst the rate of heat conduction within the bulk is low, it isn’t nil, and gradually the volume of DRI involved with what is now referred to as a “metal fire” increases. It may pass from hold to hold through a bulkhead. It may overheat fuel in adjacent tanks.

As I said, this article is not going to go into detail about what the different types of DRI are and how they should be carried. I will say, however, that one of the main tools in the arsenal of a vessel carrying DRI is the ability to exclude oxygen from the stow. This may be enough to prevent a fire in the first place, or it may help in keeping a situation under control so the vessel can safely reach a port where the problem can be dealt with. Nitrogen gas is used to inert holds carrying some types of DRI precisely for this purpose. Unfortunately, the more commonly found carbon dioxide inert gas is not suitable.
When DRI gets to high temperatures, it becomes a very difficult commodity to deal with. Hot DRI really loves oxygen, and will do almost anything to get it. Nitrogen is effective in stabilising where the hatches are closed, but oxygen will inevitably get into the stow during discharge. Temperatures can get very high indeed and there can be a risk to the vessel itself.
The only recourse available to assist during the discharge of a problem DRI cargo is to use water. Even that is fraught with difficulty. There are multiple problems. Firstly, problematic DRI can be at several hundreds of degrees or more. Initial amounts of water will simply be boiled off as steam. You have to be able to put water in there quicker than the DRI can boil it off.

Next problem is that water is H2O, and that O is of course oxygen. Hot DRI can satisfy its need for oxygen directly from the water you are pouring on it. The DRI takes the O, creating more heat and leaving the H2. This is hydrogen gas, very volatile and highly flammable. Be ready for whooshes of flame from the hydrogen, assuming you successfully avoid an explosion.
And then there is the fact that you are putting water, possibly even sea water, onto DRI. Any thought you might have had of salvaging any of the cargo is probably history by now, but there is a very real problem of creating further heating in stockpiles (hopefully ashore) from wetting by extinguishing water.
If all of that wasn’t enough, the final insult is that that the resulting oxidised DRI will weigh about 30% more than the DRI cargo you started with. Anecdotally, problems involving the collapse of quay structures have been caused by underestimating the weight of the reoxidised cargo.
Phew. If you are going to carry DRI, it really is very important that you don’t have a problem. But DRI can cause difficulties even when there isn’t an emergency/fire situation. What might they be?
Inevitably during loading and discharge, dust is created. That dust is metallic iron. The IMSBC Code warns about the need to remove accumulations of dust as quickly as possible. I have seen first-hand what happens if you don’t or can’t. Accumulations of DRI dust embed themselves into paint and then rust. This is not only unsightly but it can cause significant operational problems. The Code also warns about the potential for damage to radio and other aerials/antennae.
All in all, carriage of DRI is not something to be embarked upon without a great deal of preparation and care. At Sheard Scientfic, we have first-hand experience of dealing with carriage and consequences of carriage of DRI and can assist our clients with this highly problematic material.
A lasting feat of engineering

For as long as I can remember, the Settle-Carlisle railway line and the stations it served have been threatened by closure. This includes, famously, the amazing Ribblehead Viaduct. Begun in 1869, it was a necessary part of the Midland Railway’s project to have their own route to Scotland. A fabulous sight, whether from Ribblehead Station, from underneath the viaduct, whether it is being traversed by modern rolling stock or British Railways 46100 Royal Scot with a rake of historic Pullman coaches.




Biotechnology and GMOs – Part 2 – what is PCR?
In recent weeks, a number of P&I Clubs have circulated an information document kindly produced by Istanbul Correspondents Vitsan. This document warns that Turkish Courts have banned the import of distillers dried grains & solubles (DDGS) cargoes containing a particular genetically modified maize variety (MON810, more about that later).

The document warns that paperwork needs to be in order regarding shipments of DDGS being imported to Turkey. It seems likely that some form of testing on arrival may take place, and that is going to use a test known as PCR. But what is it?
PCR is the Polymerase Chain Reaction. There you go – shortest post ever!
Ah, you want to know how it works and what it does? OK, step this way. All through the weekly COVID Government updates, we heard the experts and Ministers use this acronym repeatedly. Before the advent of cheap lateral flow tests, it was the only way to establish if someone had that infection. It remains the way of investigating which variant of the virus an individual has, demonstrating when new variants appear, and tracking the spread of those variants. Whenever you see DNA testing referred to on CSI/forensics TV shows, PCR is the technique they are applying.
As a tool, it has revolutionised molecular biology, and it is difficult to imagine modern genetics being developed without it. That is remarkable bearing in mind it was only developed in the 1980s.
There’s no substitute to understanding the basics of how it works. Fundamentally, it selectively reproduces and amplifies a specific segment of DNA, if it is present.
I discussed in a previous post what DNA is and how it codes for information. DNA exists as a double-helix, with the gaps between the backbones occupied by base pairs AT, TA, GC and CG. This is referred to as double-stranded DNA. Each sugar backbone molecule (dexoyribose sugar) is joined to the next sugar in the same way – carbon 5 on one molecule is joined to carbon 3 on the next. The two helices run in the opposite directions and are joined together by the base pairs.
When DNA is heated up, the two strands separate. This is sometimes called “melting”, and it happens at a temperature of around 94-98oC. The two strands, now separated from each other, both contain the full genetic information the DNA coded for. That is because each A on the top strand was originally paired with a T on the bottom, each G with a C and so on.
This means that if there was a mechanism of adding new bases and deoxyribose sugars to a single strand of DNA, the full double stranded molecule could be recreated. This is what happens in cellular division in real life – the double-stranded DNA is separated into two single strands (not by heat in a cell, though!) and the cell then creates new matching strands leaving it with two identical DNA molecules. This replication process is where errors can arise resulting in genetic mutations, but this article isn’t the place to go into that.
So, inevitably, cells already have the equipment to synthesise a replacement strand to convert single-stranded DNA to double-stranded. This equipment is an enzyme known as a DNA polymerase. It needs a supply of free bases and dexoyribose sugar backbones to work.
It also needs something else. DNA polymerase requires a segment of double-stranded DNA in order to start. Once it has started, it will continue literally up the existing strand of DNA, manufacturing a complementary strand to produce double stranded DNA. Think of it as being like a zipper – you have to start a zipper by aligning the starting pieces. DNA polymerase works along like a zipper but it manufactures one side of the zip as it goes.
In a PCR reaction, short segments of single stranded DNA sequences known as primers are put into the reaction vessel. These are designed to be a perfect match for part of the DNA being tested for. Two primers are used – one matches a sequence on one strand of DNA, the other matches a sequence on the other.
In order for the primers to settle and bind on the sequences they match, the temperature has to be lower than the 94-98oC I mentioned above to separate double-stranded DNA. Typically this happens around 50-65oC. The primer sequences then act as the starter for the DNA polymerase to start and zip from.
Most DNA polymerase enzymes are not sufficiently thermally stable to survive being heated to 94-98oC in the first step. Such enzymes would need to be added, and then the “zipping”/elongation stage is usually run around 72-75oC. However, a crucial discovery for the development of PCR was the existence of a DNA polymerase from the organism Thermus aquaticus, which can survive at the 94-98oC required to denature DNA. This enzyme is known as Taq polymerase after the organism it was extracted from.
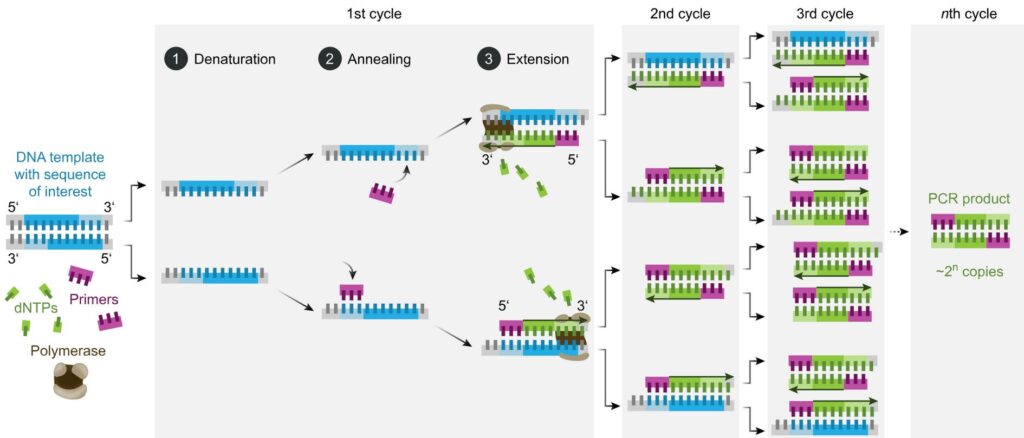
PCR is a reaction run in cycles. A reaction vessel is used into which are placed the DNA to be tested, primer sequences, base pairs on deoxyribose sugar backbones, and Taq polymerase. The reaction proceeds as follows (leaving out initial and final temperature changes which are significant but outside the scope of this article).
- Denaturation. 94-98oC for 20-30 seconds. Long enough to separate the double-stranded DNA to single stranded but not long enough to destroy the Taq enzyme.
- Annealing. 50-65oC for 20-40 seconds. In this time the primers bind to DNA sequences on the target DNA, if those sequences are present. Choice of primers and temperature are crucial – see below.
- Elongation. 72oC. Required time varies. In this period the Taq polymerase synthesises a new strand of DNA starting at a primer and running onwards until it reaches the end of the DNA strand or runs out of time.
- Repeat
The reaction is controlled by the temperature – thus the reaction tubes are placed in a machine which cycles the temperature through the regime listed above. Each full cycle takes a couple of minutes. In an hour or so, it is straightforward for 30 cycles to be run without any more reactants needing to be added to the vessel or any other intervention.
As mentioned above, PCR is used to amplify in a selective manner a particular fragment of DNA. Assume that we are testing for the presence of MON810 maize DNA, and that the sample being tested does indeed contain that DNA – but only one molecule of it. (Potentially bad news if you are importing that maize to Turkey). The denaturation step will create two single-stranded segments of DNA from the extracted maize sample. These will not be the same but one will be effectively the reverse/mirror of the other. One primer must be designed to be a match/complement to a DNA sequence on one strand, and the other primer to a different area on the second strand. They bracket the DNA sequence to be amplified.
On the first cycle, one primer will bind to each strand of the sample DNA, and those will be elongated in step 3. Note that the DNA sections extracted from the maize sample might be very long indeed. The elongated copies made in this first step 3 will start at a primer (because that is how Taq polymerase works) and will extend for some distance until the point where the polymerase ran out of time in step 3.
On the second cycle things become interesting. Denaturation will produce four single-strands of DNA. Two of these will be the original ones from the sample (of whatever length they were) but the other two will start with one of the primers and will then be of random length. Call the two primers P1 and P2.
The strand synthesised in the first reaction starting with P1 doesn’t contain a sequence P1 can bind to – so only P2 can bind. Similarly, the strand which started with P2 in the first cycle will then bind with P1. Elongation in the second cycle will produce double sided DNA from all four single strands. Two of these (the ones produced from the synthesised segments in cycle 1) will have a new segment synthesised which starts at P1 and stops at P2. That is the desired amplification product.
At the end of the second cycle, we are left with four double stranded segments of DNA. When denatured at the start of the third cycle, six of these (i.e. the two original DNA strands, the two synthesised in cycle 1, and two of the four synthesised in cycle 2) are what is known as variable length strands. The other two however are exactly the same length as each other, and that length is the number of DNA base pairs between the sequences in the original DNA which bind to P1 and P2.
This might not sound so great, as we have three times as many DNA fragments of variable length than the specific sequence we were intending to multiply.
However, at the end of this third cycle, we have 16 strands of DNA. Eight of these are variable length fragments (two new variable length fragments were synthesised in cycle 3 from the original sample DNA). However, the other eight are exactly the same length – each starts at the location of P1 and runs to the location of P2.
At the end of the fourth cycle we have 32 strands of DNA. Ten are variable length, but all of the rest are exactly the same – starting at P1 and ending at P2.
However, now run the reaction cycle a further 29 times. You end up with a couple of hundred variable length fragments and over 1,000,000,000 (one US billion) identical copies of a fragment exactly the length bounded by P1 and P2. The enormous quantities of exact length fragments completely swamp the variable lengths fragments.
The resulting contents of the reaction tube are then visualised in some way so that the results can be understood. They might be separated on a gel – this is how the charts are produced showing the characteristic lines we see in DNA results. Each line is an amplified fragment – a fragment bounded by two specific primers. These amplified fragments, even from a tiny original amount of DNA, are present in such enormous numbers they are readily detected and visualised.
To return to our example, if the sample of maize DNA didn’t contain a fragment from MON810, that fragment couldn’t be amplified, and that “line” would be absent on the chart.
Everything then depends on the selection of suitable primers (and to some extent, the fine-tuning of the temperatures used in the PCR cycles). The primers need to be long enough that there is no chance of those sequences being found elsewhere in the maize genome, and they need to be short enough to bind with the target DNA during step 2 of each cycle – if they don’t then there is nothing for Taq polymerase to zip from.
As an example, the EU reference test for MON810 uses the following primers
- P1 – TCGAAGGACGAAGGACTCTAACGT
- P2 – GCCACCTTCCTTTTCCACTATCTT (reverse strand)
This test is known as an “event specific” test because primer P1 binds to a sequence on the maize parent plant whereas P2 binds to a sequence on the inserted gene – i.e. the fragment to be amplified contains a section of DNA from the maize and a section from the insert, it flanks the genetic insertion. Thus this test only detects MON810, it doesn’t (for instance) detect other GMOs which contain the same inserted DNA.
More on the use of PCR to detect GMOs in relation to shipping in a later article.
For now, I will offer some comments on the generalities of using PCR and the MON810 variation.
The enormous specific amplification provided by even 30 cycles of PCR means that very tiny amounts of DNA can be detected. Provided the primers are appropriately selected, the test is wholly specific – it only detects the sequences it was designed to detect. There are off-the-shelf primer kits available for commercially produced GMO plants. Thus an event specific test can be anticipated to exist for any GMO likely to be found in a real world shipment.
As long ago as 1999, methods for detection of GMOs were being studied in the context of commercial commodities. This is because of the fear which has existed for some time of “Frankenstein foods” and other issues relating to escape of modified material into the natural environment. One particular study looked for a specific GMO soya type (commonly known as Roundup-Ready soya) not in samples of soya but in wheat bread. Soya was only in the bread as a minor component of the baking improver/powder. Despite the steps making up the dough production and baking, GMO soya was readily detected in loaves produced using that baking aid – even though the soya made up only 0.4% of the dough mix. In that study, commercial baking aids were also tested, and in 2 out of 15 off-the-shelf products tested the GMO soya could be detected in the baked bread. Back in 1999!
And so, by a roundabout route we return to maize and MON810. MON810 is quite an early GMO product of the Monsanto company. It was made by inserting DNA from a bacterium Bacillus thuringiensis. In the bacterium this produces a toxin which affects certain butterflies and moths, some of which as larvae damage maize plants. The idea of the modification was that the maize plants would produce the toxin, and that would serve as a control over the larvae, thus reducing the need to apply pesticides. MON810 has been commercially very successful and has been very widely planted. The modification was produced by firing projectiles containing the bacterial DNA at plant cells.
MON810 has been the subject of some controversy. It has been suggested that the use of this variety can be responsible for the death of bee colonies. As far as I am aware, this allegation, or indeed any other specific allegations of harmful effects from MON810 has not been proven.
As stated above, the EU test for MON810 is event specific and will detect very small amounts of the GMO. Even a fragment from a MON810 plant will, if it ends up in a sample sent for testing, lead to a positive result. I note that the Vitsan circular relates to imports of distillers dried grains & solubles (DDGS) – not the maize itself. DDGS is produced from harvested maize by a series of industrial steps, but by analogy to the baking powder/bread study referenced above, these processes are not likely to prevent detection of modified genetic material if MON810 was present in the original unprocessed maize.
I note that Vitsan have specifically clarified that a different product, NK603xMON810 maize is allowed to be imported, provided it is referenced as MON-00603-6xMON-00810-6. The “x” in this designation identifies that this is a so-called “stacked trait”. This means that the maize variety MON-00603-6xMON-00810-6 carries the properties of MON810 and the properties of MON603. The GMO event 603 is a Roundup-Ready variety of maize. Thus the stacked trait is resistant to the herbicide Roundup (glyphosate) and also produces the bacterial toxin to kill the larvae mentioned above.
Often, but not always, stacked traits are produced by conventional genetic crossing techniques. If this is done, and plants which express both traits are selected for, then BOTH events will be present in the marketed product. Thus a test for event MON810 will be positive, as will a test for event 603. It is not clear to the writer what undesirable properties could exist in MON810 which are not also present in MON-00603-6xMON-00810-6. I do not have sufficient data yet to say whether MON-00603-6xMON-00810-6 will test positive in an event specific test for MON810.
It appears likely that there may be difficulties with testing surrounding verification for this regulation – i.e. cargoes of DDGS may be rejected when they should not be. The presence of genetically modified material is not something which can be detected by the naked eye nor even field testing. It requires sampling and laboratory analysis, and there is always scope for a misleading result.
A new Code – Part 2
So what’s new?

Starting with the Supplement, there is a new document entitled “Guidance for conducting the refined MHB (CR) test”. MHB materials are those with hazards which are not to be found in the IMDG Code as they are only considered hazardous when in bulk form. The application of MHB has changed in approach in recent years, and I will write about that separately.
The new document to be found in the Supplement relates to extending the scope of a UN Standard test (known as the C1 test) to apply to examining the corrosivity of bulk solids. The document notes that the C1 test in its standard published form does not give reliable results for bulk solids. The new guidance runs to several pages and is in effect a modification or adaptation to the C1 test enabling it to be conducted on bulk solids. This fortunately isn’t something most vessels will need to encounter.
Also in the Supplement is a new version of the document issued as circular 1395. This document contains “Lists of solid bulk cargoes for which a fixed gas fire-extinguishing system may be exempted or for which a fixed gas fire-extinguishing system is ineffective”. This document has been updated to include the materials to be found in the new Code schedules – see below. I note that this document relates to what are usually referred to as CO2 systems on ships. The CO2 system is frequently one of the first tools reached for by vessels undergoing a fire emergency. The first list in this document is made up of materials which are deemed very unlikely to support a fire. The second (for which a CO2 system is ineffective) is entirely made up of materials which are chemically inorganic nitrates. These are oxidising agents. As CO2 works by excluding oxygen and thus suppressing combustion, it is of no use for a cargo which contains its own oxidising agent.
Turning now to the main Code, a change which is really a matter of housekeeping from previous editions is the embedding of the concept of dynamic separation in the definitions and wording of parts of sections 1-13 of the Code (i.e. the bit before the appendices start). If you read my introduction to liquefaction, you may recall that dynamic separation as a phenomenon had been described in the new bauxite fines schedule. That schedule is of course to be found in Appendix 1 of the Code. No definition of dynamic separation existed in the sections of the Code discussing liquefaction, nor was there any wording to make it clear that Group A properties included both liquefaction and dynamic separation. Now there is.
The heading of Section 7, for instance is now “Cargoes which may liquefy or undergo dynamic separation“, whereas it was previously “Cargoes which may liquefy“. Similarly, the tests in Appendix 2 are now (all) tests for “materials which may liquefy or undergo dynamic separation.“
There is now a definition of the term “dynamic separation” itself, which is that
Dynamic separation means the phenomenon of forming a liquid slurry (water and fine solids) above the solid material, resulting in a free surface effect which may significantly affect the ship’s stability.
IMSBC Code definition of dynamic separation, 2022 Edition
This is the Code catching up with itself – the 2020 edition contained a schedule in an appendix which outlined a hazard which wasn’t defined in the Code itself. It is now very clear that dynamic separation and liquefaction are both to be taken as examples of Group A cargo behaviour. The procedure for preventing them is the same – Group A cargoes must be shipped at a moisture content below the determined Transportable Moisture Limit (TML).
Bearing that in mind, the distinction between liquefaction and dynamic separation only becomes significant when a cargo misbehaves. The emergency measures taken by a vessel may need to take into account whether the cargo is considered to be undergoing liquefaction or dynamic separation, but the normal routine handling and carriage does not.
Finally, I will turn to Appendix 1 – in this we find some new schedules and one commodity re-classified.
LEACH RESIDUE CONTAINING LEAD. This is a new schedule. It relates to solid residue produced during zinc extraction. Sulphuric acid is used to dissolve the zinc-bearing part of the ore or concentrate. What is left may contain lead (resulting in toxicity) and may be corrosive because of residual sulphuric acid. I note that this schedule is MHB TX and CR – the CR corrosive solid MHB hazard will require the new modified corrosivity test to be found in the Supplement. A Group A cargo on account of its particle sizing (granular), and a Group B cargo on account of the two MHB hazards.
CLAM SHELL. Another new schedule. Other than noting that it is Group C and relates only to whole clam shells, I have nothing to say!
SUPERPHOSPHATE (Triple, granular). This is a replacement schedule. In the previous edition of the Code, TSP (as it is usually known) was listed as a Group C cargo. The new schedule is a Group B schedule on account of it being a corrosive solid MHB. I note again that this requires the application of the new test to be found in the Supplement. TSP is the “ultimate” in a series of fertilisers obtaining fertilising phosphorous content (the P of NPK) manufactured from phosphate rock. Phosphate rock contains insoluble calcium phosphate, which is brought into solution by reacting with an acid. Sulphuric acid can be used which results in superphosphate. TSP has the highest amount of phosphorus in it, and it is made by reacting phosphoric acid with phosphate rock – the resulting material has phosphorus from both the phosphate rock and the phosphoric acid. Residual acidity results in the MHB CR designation.

… and finally, AMMONIUM NITRATE
There were four schedules for ammonium nitrate and material derived from it in the 2020 Code. They were
- Ammonium nitrate UN1942
- Ammonium nitrate based fertiliser UN2067
- Ammonium nitrate based fertiliser UN2071
- Ammonium nitrate based fertiliser (non-hazardous)
Of these, the “non hazardous” version was Group C and the first three were Group B cargoes. Note that ammonium nitrate in conjunction with combustible material such as fuel oil is highly explosive. One of the requirements to be considered non-hazardous was that the material passed the UN standard test known as the “trough test”. I wrote about this test and some of the problems arising from it earlier this year. I commented that the trough test did not fully reproduce the hazard of self-sustaining decomposition meaning that cargoes which were tested and certified as “ammonium nitrate fertiliser (non-hazardous)” could give rise to serious incidents.
I have, over the years, been consulted many times regarding proper classification of materials containing ammonium nitrate. I suspect the new provisions, whilst being technically more complete and consistent than the previous version, will also result in queries. That is because they are even more complicated.
In the new Code, 2022 edition, we now have (in the order they appear in the Code)
AMMONIUM NITRATE UN1942. This schedule is unchanged from previous versions which covers ammonium nitrate itself. It is a Group B schedule, Class 5.1 oxidiser and is an extremely hazardous substance. The schedule sets very strict limits on the amounts of combustible material.
AMMONIUM NITRATE BASED FERTILISER UN2067. This is also Group B, Class 5.1 and is carried over from previous versions. There are compositional limits set for this category of fertiliser derived from ammonium nitrate which result in it being less hazardous than the UN1942 material.
AMMONIUM NITRATE BASED FERTILISER UN2071. This is also carried over from previous Code versions and is a Class 9 Group B material. This schedule applies to ammonium nitrate based fertilisers with compositional limits set to result in material less hazardous again than UN2067 or 1942. The substance is no longer sufficiently oxidising to result in a Class 5.1 designation but is Class 9 because it can undergo self-sustaining decomposition. This and the remaining two schedules are where the new provisions start to become somewhat complicated to follow. A fertiliser which passes the UN Trough Test is not subject to the provisions of this schedule.
AMMONIUM NITRATE BASED FERTILISER. Note that the designation “non-hazardous” is no longer present. This is a Group C schedule which, together with the MHB schedule below replace the old “non-hazardous” schedule. The Group C schedule says it applies to mixtures which fall within certain limits defined by the amount of ammonium nitrate and the amount of chloride present. To be within the Group C schedule, the material has to fall within the grey shaded area in the graph reproduced below. Note that either the amount of ammonium nitrate is very low or the amount of chloride is very low (or both).
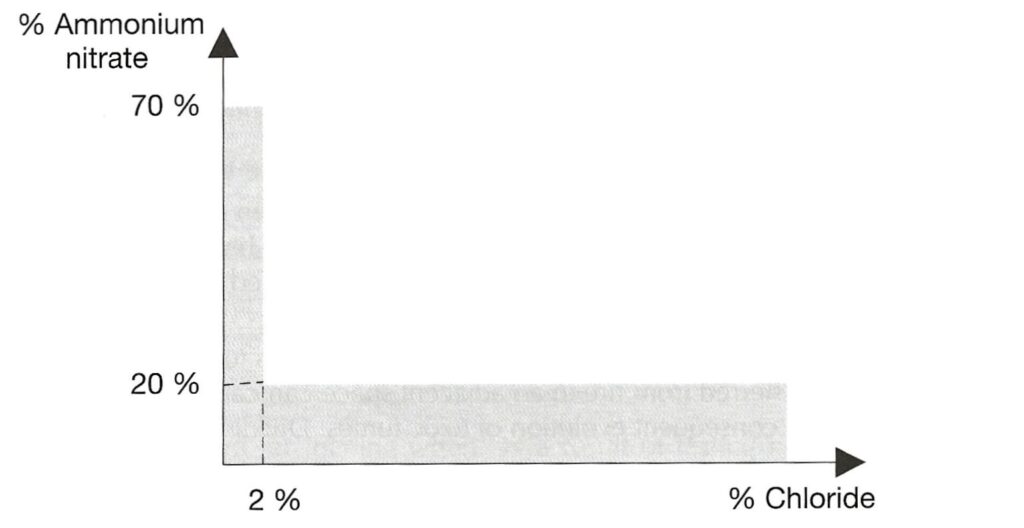
There is a provision in this schedule that materials which fail the trough test are excluded from the schedule. It is a stated hazard that Group C ammonium nitrate based fertiliser will decompose if exposed to heat, but that decomposition is “highly unlikely” to spread through the bulk.
AMMONIUM NITRATE BASED FERTILISER MHB is the final schedule, and this is wholly new. It is categorised as Group B, hazardous only in bulk (MHB) of type “OH” (other hazard). This means a hazard which is not one of the standardised ones. The schedule includes a definition in terms of compositional basis to distinguish it from the Group C material above. The graph is the complement of the one reproduced above – i.e. it covers material with more ammonium nitrate or more chloride or both but not sufficient ammonium nitrate to make the material one of the more hazardous types.
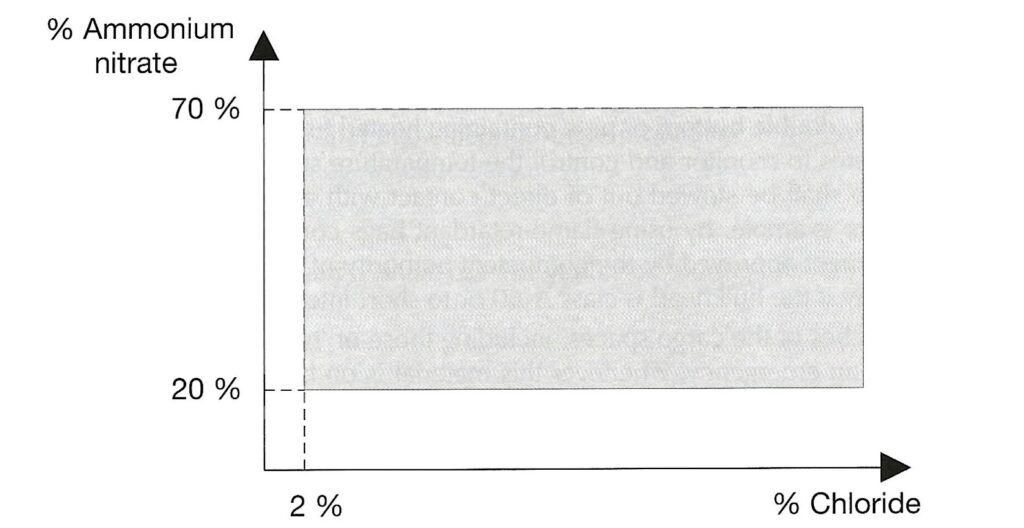
Again, the schedule states that material which fails the trough test is excluded. This MHB schedule only applies to substances which pass the trough test. However, this schedule states that this material has the hazard that an initiated decomposition event can propagate through the bulk.
This new schedule then accommodates ammonium nitrate fertilisers which pass the trough test but do actually possess the hazard the trough test was designed to detect. The schedules appear to have been written in this manner as a work-around for the fact that the trough test does not detect all fertiliser mixtures which can undergo self-sustaining decomposition.
I note that historically, research has shown that self-sustaining decomposition is only a possibility with a mixture which includes ammonium nitrate and chloride (generally NPK fertilisers where the K element is supplied by potassium chloride/muriate of potash). That is the basis behind the graphical composition criteria.
Working backwards, the effect of the new provisions is as follows. Firstly, materials which are compositionally UN1942 or UN2067 remain covered by those schedules. Any other fertiliser material which contains ammonium nitrate and fails the trough test is Class 9 UN2071.
The remaining two schedules apply to any substances which pass the trough test, and which one applies depends on the levels of ammonium nitrate and chloride. The implication of the criteria is that ammonium nitrate fertiliser with below 20% ammonium nitrate or below 2% chloride can be assumed not likely to undergo self-sustaining decomposition and can be consdered Group C. The MHB schedule covers the rest, and presumably would have applied to the CHESHIRE cargo and the PURPLE BEACH cargo had the new schedules been in existence at the time those cargoes were shipped. [Note that I am not party to technical data regarding the cargoes on these two vessels but assume this to be the case.]
How does this new set of schedules benefit the seafarer? Well, the MHB schedule is the one to focus on because it encompasses substances which were prevoiusly categorised as non-hazardous despite being capable of leading to the total loss of a vessel such as CHESHIRE. The big difference is that the seafarers will be aware of the potential risk and will therefore be more likely to detect it early if it happens. The Code outlines a number of precautions to be taken when MHB ammonium nitrate based fertiliser is carried, and some provisions indicating what to do if a decomposition event is detected during the voyage.
Hopefully the new schedules will assist in avoiding these horrific events.

A new Code – Part 1
It has been a while since I wrote here. Things have been madcap busy at Sheard Scientific. There’s just enough time now after digesting another turkey lunch for me to digest the contents of a new edition of the IMSBC Code, which landed on our doormat here at Sheard Scientific this month. I will start with this article by outlining what is in the Code and how it all works. Tomorrow, I will set out what has changed.

This tips the scales at a hefty 2.2kg. Quite a weighty document – the bulky code perhaps!
There is of course a reason for this. When the old BC Code morphed into the first version of the IMSBC Code and became mandatory under SOLAS, it contained schedules for individual bulk cargoes and a mechanism for missing or incorrect schedules to be identified and added. Those processes have been in place now for a number of years, and on each new edition, the Code gets bigger and bigger, and heavier.
The concept was that any cargo carried in bulk (other than grain cargoes, and many don’t properly understad why they are not covered) will have its own schedule. In that schedule will be a listing of the properties and hazards of the material and guidance regarding carriage.
The amendment timetable has been disturbed a little by Covid. The new edition is now available in print, and can be applied on a voluntary basis from 1st January 2023 and becomes fully mandatory on 1st December. This pattern – of publication followed by a period of voluntary applicability and then full force has been the case for previous amendments.
It is important to note that the concept of “voluntary” applicability is voluntary in the sense that the relevant Competent Authority/participating governmental body to IMO can decide that the new Code applies. It is not left to be a voluntary decision by individual shipowners, ship Masters, or indeed cargo shippers.
As the amendments to a document such as the IMSBC Code by definition represent best practice in the industry, it should in my view be in everyone’s interest for the amendments to be adopted as soon as practicable.
It is worth taking a quick look through the material making up the Code as published. Some things are in surprising places – this is to an extent a legacy of a shake-up of organisation as the material which used to be in various locations in the BC Code is mostly still to be found, just not necessarily where you might think it would be.
Sections 1-13 are where procedures, rules and regulations, and definitions are to be found. Material specifically relevant to liquefaction for instance can be found in sections 4, 7, 8, but you might also need to check section 5, which relates to trimming. There really is no substitute to reading through these sections carefully even if just to know what can be found where.
The next part of the Code is the appendices. Unlike most books, the appendices to the IMSBC Code are a fundametal part of the contents. Appendix 1 on its own is nearly 400 pages long, and contains the individual schedules for bulk cargoes, listed alphabetically by Bulk Cargo Shipping Name. These schedules are the part of the Code which a vessel’s Master will spend most time looking through, and this is the part of the Code which enlarges on each amendment as additional schedules are added.
Appendix 2 contains test methods applicable to bulk cargoes. This includes all six tests for Transportable Moisture Limit along with other tests.
Appendix 3 is headed “Properties of solid bulk cargoes” and is a curious mixture of seemingly unconnected but very important sections. One important point to bear in mind is that the contents of this appendix are not legally mandatory under SOLAS whereas some other parts of the Code are.
- Appendix 3 point 1 is a list of non-cohesive cargoes (as contrasted with other cargoes which are considered cohesive) and some provisions which flow out of this property.
- Appendix 3 point 2 is a single paragraph which describes the types of cargoes which might undergo liquefaction or dynamic separation. This paragraph is often quoted in disputes, so I will quote it here.
Many fine-particled cargoes, if possessing a sufficiently high moisture content, are liable to flow. Thus any damp or wet cargo containing a proportion of fine particles should be tested for flow characteristics prior to loading.
IMSBC Code Appendix 3 paragraph 2.1
- Appendix 3 point 3 is a provision which states that where a Competent Authority has to be consulted prior to shipment (i.e. substances not listed in the Code or substances with hazards which differ from that listed in the Code), the corresponding authorities at the loading and discharge port should be consulted. This is to ensure that all potentially involved in the carriage of a substance with chemical hazards are aware and agree with the precautions being taken.
Appendix 4 is the Index. However, it is much more than just an index. As stated above, the list of schedules in Appendix 1 is arranged alphabetically and so acts as its own index to an extent. In Appendix 4, the Index also includes entries which cross-reference to a BCSN. These can be found in lower case entries in the Index whereas entries in capital letters are the BCSNs themselves. As an illustration of how this works, the fourth entry in the Index is ALUMINA HYDRATE, a Group A&B cargo. The capital letters denote that ALUMINA HYDRATE has a schedule under that name in Appendix 1. Lower down on the same page can be found Aluminium hydroxide. As this is in lower case, it isn’t a BCSN and there is no schedule in Appendix 1 for Aluminium hydroxide. However, the entry says “see ALUMINA HYDRATE” and so the reader is thus informed that aluminium hydroxide and alumina hydrate are synonymous from the viewpoint of the entries in the Code. There are many other examples of this type of cross-referencing in the index.
Appendix 5 is similar to an Index but contains BCSNs from the Code in English, French and Spanish.
The rest of the book as published is the Supplement to the Code, and this amounts to a further 150 pages.
The documents making up the Supplement are all also published separately in their own right or as IMO circulars. They cover a wide range of topics, starting with the BLU Code (Code of Practice for the Safe Loading and Unloading of Bulk Cargoes) and ending with an alphabetic list by country of the designated Competent Authorities.
More or less in the middle of the Supplement can be found the document “Recommendations on the safe use of pesticides in ships applicable to the fumigation of cargo holds” which is also published as IMO Circular 1264. This is a vitally important set of guidelines and recommendations which is essential reading for any becoming involved with shipboard fumigation, whether that is carried out in port or when continued in transit. I mentioned this circular earlier this year in the context of a serious incident involving fumigation.
Season’s Greetings

Sheard Scientific would like to wish all of our friends and valued clients a very happy Christmas and a prosperous New Year.








































