Independence Day – a celebration!

Wow. Difficult to believe it but it is now exactly one year since I left my former employer and Sheard Scientific came into being. I didn’t start work on cases straight away, nor did I establish this website, but this is the day.
I could go on at length about working independently – the benefits to my family, health and general wellbeing are immense – but they are obvious. I am able to work from home so have no fixed days and no commute, enabling me to use that time productively and am usually available whenever I am needed by clients.
By coincidence, today is also the wedding anniversary of myself and Mrs Sheard. When I was originally recruited by John Kirman many years ago, he told me that business partnership was as close as being married to someone. I understand what he meant, but respectfully disagree. Marriage involves pledging to support and be supported by someone for the rest of your natural lives.
At no point has that been clearer to me than in 2021 when Mrs Sheard got me to hospital sufficiently quickly to save my life; she then went on to deal with everything and everyone until I recovered. At that point, she made it clear it didn’t matter if I never worked again but, if I chose to, I could do anything I wanted.
The result is Sheard Scientific. Happy anniversary!

Sugar cargoes – a visit to the Museum of London Docklands

Having been in London to attend an analysis at the GAFTA laboratory Salamon & Seaber, we paid a visit to the Museum of London Docklands, to be found in the shadow of Canary Wharf at West India Quay.
It’s a fascinating museum with mock-ups of the London docks in their heyday, information on equipment and the working practices. There’s also a whole section on the effect of the second world war and the bombing of the docks. Well worth a visit.

What caught my eye as a cargo scientist was an area on sugar. Much of this focussed on the terrible history of slavery associated with sugar production. This was fascinating and sobering, and certainly told me much I had not previously been aware about regarding that trade. There was also information about the cargo itself and its production, one form of which is still imported into London docklands to be refined. That cargo is shipped in large quantities and will be the topic of this article.
“Sugar” can refer to one of a large range of chemical entities falling under the heading “sugars”, but when it is used to refer to a cargo on a ship, that cargo will be sucrose sugar, which is the substance used to sweeten tea and coffee, and in baking. Sugar crops are grown as sugar cane in the caribbean and elsewhere, and sugar beet in temperate climes. Once the sugar has been extracted from the parent plant, its origin (i.e. whether it came from beet or cane) is less significant than how much and what type of processing or refining it has been subjected to.
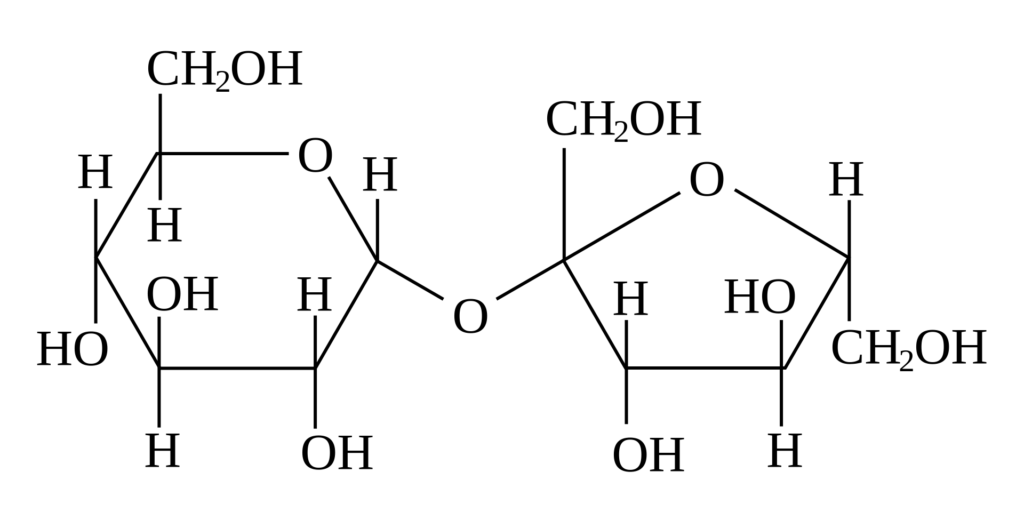
If this molecule is broken in the middle, two smaller sugars are produced – the left one is glucose (the fundamental fuel for cellular life) and the right one becomes fructose (the sugar found in fruit). That splitting happens in real life by reaction with water (hydrolysis), and that is a very important process to understand.
Sugar is carried by ships as one of two main types – raw sugar, which is usually carried in bulk, and refined sugar, which is usually to be found in bags. For the purposes of carriage, these behave very differently and require different consideration from the carrying vessel.
Raw (bulk) sugar
This, as the name suggests, is sugar which has received relatively little processing. It is off-white or darker in colour, granular, but doesn’t flow readily. The most important thing to bear in mind when dealing with raw/bulk sugar is that it will be intended to be subjected to refining prior to use. This means that raw sugar is one of the least sensitive cargoes when undergoing carriage at sea.
Even sugar from a hold which is submerged in water will contain substantial value. There are a number of consequences of water ingress into bulk sugar. One of these is simply that the sugar contains water. Thus a tonne of cargo discharged will contain less actual sugar than a tonne of the cargo did when it was shipped. This isn’t a real loss, but it does make it more difficult to keep track of the quantities of cargo being discharged and handled post-incident. I have seen severe incidents, where a hold has become tidal, where some of the sugar/syrup has washed out of the hold. That of course is a real loss, and it then becomes very important to keep track of how much sugar is in the various parcels eventually off-loaded.
The presence of water or excess moisture will also accelerate hydrolysis reactions as mentioned above – some of the sucrose di-saccharide molecules will react with water to produce glucose and fructose molecules. These will be removed in refining, meaning that the amount of refined sugar produced will reduce. This is also a real loss, but it is evaluated in a different way.
I discussed the basic equations of electromagnetism (Maxwell’s equations) a while ago. To explain how damage to bulk sugar is assessed, I need to talk a little about polarised light. The Poynting vector points in the direction the ray of light is travelling, but the electric and magnetic fields which make up the light wave are at right angles to this vector (and to each other). Thus a light bean travelling directly away from you may have electric field directed vertically up/down and magnetic field left/right. Most sources of light are a combination of differently oriented electric and magnetic fields. Plane-polarised light has each of these fields only present in one direction. Polarised light can be created by the light source but it can also be created by polarising or “polaroid” sunglasses. Thus a non-directional light source such as light from the sun outside can be made into polarised light by passing it through a polaroid filter. As this cuts out all light oriented in the perpendicular direction, it reduces the intensity of the light passing through. That’s why polaroid sunglasses help on bright sunny days.
Once you have created polarised light by passing through a polaroid lens, it remains polarised with that orientation until something else happens. If you pass that light through a second polaroid lens, what happens depends on the orientation of the second polaroid. If the two polaroids are lined up, the light will pass straight-on through the second. If they are oriented at 90° to each other, there will be no light at all passing though the second lens. Try it. Hold two polaroid lenses (perhaps two pairs of sunglasses) up to the light and rotate the second pair relative to the first. You will see it go successively light and dark as the lenses line up.
What’s that got to do with sugar? Good question. Sugar solutions have the property that they can rotate the orientation of plane-polarised light. So if you have your two polaroid lenses at 90° and shine a light through (any light), you get no transmission through past the second lens. Put a sugar solution in between the lenses, you suddenly get light transmission again. To get no transmission, you would have to rotate the second polaroid. The angle you would have to do that by is a measure of how much the sugar solution rotated the plane polarised light.
A test carried out with a sample of sugar will make up a solution in a prescribed manner and establish the angle by which that solution rotates plane polarised light. From this is calculated a number called “pol”, which is expressed as a percentage. Pure sucrose has a pol of 100%.
Adding water reduces the pol (but as stated above doesnt actually reduce the amount of sucrose. Hydrolysis of sucrose to form glucose and fructose not only reduces the concentration of sucrose (and thus reduces the pol for that reason) but, because fructose rotates plane polarised light in the other direction to sucrose, hydrolysis actually reduces the pol by more than the amount of sucrose which has been hydrolysed.
Thus, if you have a cargo of bulk sugar, it is very important to sample it properly and representatively, and then measure the moisture content and the %pol. From these figures it is possible to calculate how much damage the sugar has suffered. Usually it isn’t as much as might be imagined from the cargo appearance.
As bulk sugar is hygroscopic, it should be ventilated according to the normal rules of seamanship. Having said that, condensation isn’t going to cause any genuine problems, so it doesn’t really matter what ventilation is applied.
Refined (white) sugar
This is usually carried in bags. There are a number of different grades of refined sugar, and I’m not going to go into the differences here. As a refined product, it is usually intended for direct human consumption. It tends to have a very low moisture content and will readily absorb moisture if exposed to the atmosphere.
Traditionally, the bags are made of woven white polypropylene, and they have a plastic film inner liner, which is stitched to the outer bag structure at each end. The polypropylene provides the mechanical strength and the film liner provides a moisture barrier. The moisture barrier is not a complete one – some moisture can get through the plastic film, and also moisture or even water can penetrate the film where the stitching has made holes in it. But the presence of the liner largely separates the very dry and hygroscopic sugar from the in-hold atmosphere. This means that for practical considerations, the sugar cargo in bagged form is not hygroscopic. Bagged refined sugar should not be ventilated. There are no circumstances in which it would be necessary to ventilate bagged sugar.
Indeed, there are circumstances where applying ventilation to bagged sugar can cause damage. Two mechanisms come to mind. The first is the classic “cargo sweat” situation. If inherently cold bagged sugar is loaded and carried to a warmer climate, and ventilation is applied, that can lead to condensation directly onto the bags and wetting of the bags. The sugar will remain cold throughout even a long voyage, and warm moist external air can cause substantial condensation onto the bags, particularly if the vessel has mechanical (i.e. fan-assisted) ventilation facilities.
The second mechanism is that ventilating with air of different temperature to the bags (warmer or cold) might be said to induce temperature fluctuations which could give rise to caking. As caking is one of the biggest problems experienced with sugar, it is important that nothing is done which might promote that caking.
Folklore suggests that sugar in lined bags can come into contact with splashes of water, but that isn’t recommended. Heavy condensation or wetting as a result of water ingress will have a tendency to penetrate the bags through the stitched ends. If that has happened, a region of syrupy sugar will be found at the end of such a bag when it is cut open. Most of the water will run off the lined bags, with only a small amount gradually seeping in through the stitches. This means that a stow of sugar in bags where there is water ingress from (say) a hatch cover leakage will suffer disproportionate numbers of damaged bags as a consequnce when compared to bags of rice which absorb much more of the water in the first few tiers affected. In way of a leakage, the water will find its way downwards leaving a pyramidal structure of wetted bags. The actual amount of water damaged sugar will be relatively small but that will be distributed amongst many bags.
Problems can also occur if bags are handled roughly, as the film liners can become torn. Likewise, if stevedores use hooks to lift the bags, the film liners will become compromised.
As I have said above however, caking is the most problematic issue with refined sugar, and that does not require damage to be bags or water ingress. Many articles have been written describing caking and what sort of inspections to carry out on caked bags identified after carriage. There are things you can do to investigate – like picking bags up and dropping them, cutting bags open to see whether the caking is present throughout or just the ends, and so on.
Sugar can cake very hard indeed. That is usually associated with the sugar not having been properly conditioned after refining. If sugar is bagged without conditioning, it can be too warm, too moist, or with moisture not equilibrated. These factors tend to give rise to hard caking by means of sugar being deposited in crystalline form between two adjacent granules, binding them together. If that process happens extensively in a bag, it can result in sugar which is wholly moulded to the shape the bag was compressed into in the stow without there being any straightforward means to return the sugar to its original free-flowing nature.
If a vessel is loading sugar which hasn’t been properly conditioned, there isn’t much they can do to prevent or reduce caking. It is a good idea to get an idea of the temperature of bagged sugar at the time of loading – but not using a spear thermometer!