Sugar cargoes – a visit to the Museum of London Docklands

Having been in London to attend an analysis at the GAFTA laboratory Salamon & Seaber, we paid a visit to the Museum of London Docklands, to be found in the shadow of Canary Wharf at West India Quay.
It’s a fascinating museum with mock-ups of the London docks in their heyday, information on equipment and the working practices. There’s also a whole section on the effect of the second world war and the bombing of the docks. Well worth a visit.

What caught my eye as a cargo scientist was an area on sugar. Much of this focussed on the terrible history of slavery associated with sugar production. This was fascinating and sobering, and certainly told me much I had not previously been aware about regarding that trade. There was also information about the cargo itself and its production, one form of which is still imported into London docklands to be refined. That cargo is shipped in large quantities and will be the topic of this article.
“Sugar” can refer to one of a large range of chemical entities falling under the heading “sugars”, but when it is used to refer to a cargo on a ship, that cargo will be sucrose sugar, which is the substance used to sweeten tea and coffee, and in baking. Sugar crops are grown as sugar cane in the caribbean and elsewhere, and sugar beet in temperate climes. Once the sugar has been extracted from the parent plant, its origin (i.e. whether it came from beet or cane) is less significant than how much and what type of processing or refining it has been subjected to.
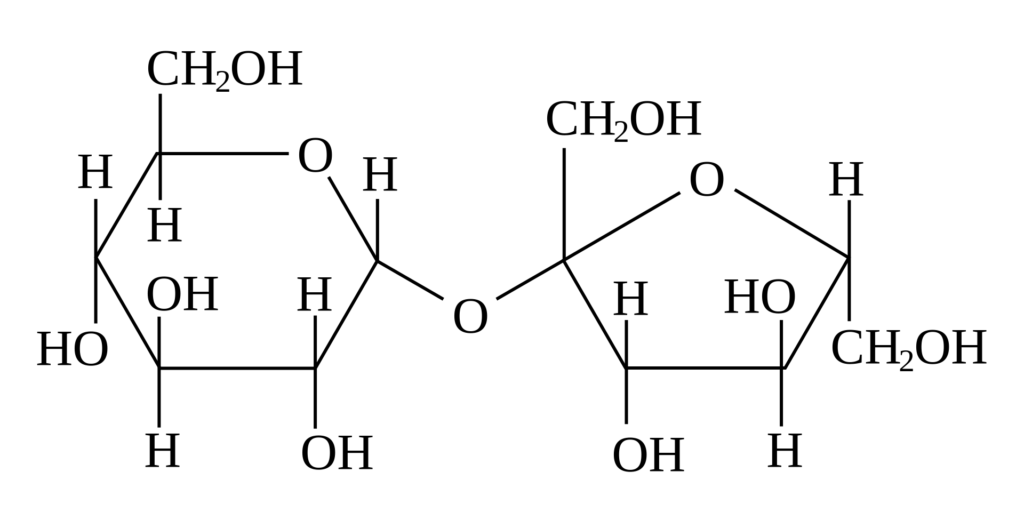
If this molecule is broken in the middle, two smaller sugars are produced – the left one is glucose (the fundamental fuel for cellular life) and the right one becomes fructose (the sugar found in fruit). That splitting happens in real life by reaction with water (hydrolysis), and that is a very important process to understand.
Sugar is carried by ships as one of two main types – raw sugar, which is usually carried in bulk, and refined sugar, which is usually to be found in bags. For the purposes of carriage, these behave very differently and require different consideration from the carrying vessel.
Raw (bulk) sugar
This, as the name suggests, is sugar which has received relatively little processing. It is off-white or darker in colour, granular, but doesn’t flow readily. The most important thing to bear in mind when dealing with raw/bulk sugar is that it will be intended to be subjected to refining prior to use. This means that raw sugar is one of the least sensitive cargoes when undergoing carriage at sea.
Even sugar from a hold which is submerged in water will contain substantial value. There are a number of consequences of water ingress into bulk sugar. One of these is simply that the sugar contains water. Thus a tonne of cargo discharged will contain less actual sugar than a tonne of the cargo did when it was shipped. This isn’t a real loss, but it does make it more difficult to keep track of the quantities of cargo being discharged and handled post-incident. I have seen severe incidents, where a hold has become tidal, where some of the sugar/syrup has washed out of the hold. That of course is a real loss, and it then becomes very important to keep track of how much sugar is in the various parcels eventually off-loaded.
The presence of water or excess moisture will also accelerate hydrolysis reactions as mentioned above – some of the sucrose di-saccharide molecules will react with water to produce glucose and fructose molecules. These will be removed in refining, meaning that the amount of refined sugar produced will reduce. This is also a real loss, but it is evaluated in a different way.
I discussed the basic equations of electromagnetism (Maxwell’s equations) a while ago. To explain how damage to bulk sugar is assessed, I need to talk a little about polarised light. The Poynting vector points in the direction the ray of light is travelling, but the electric and magnetic fields which make up the light wave are at right angles to this vector (and to each other). Thus a light bean travelling directly away from you may have electric field directed vertically up/down and magnetic field left/right. Most sources of light are a combination of differently oriented electric and magnetic fields. Plane-polarised light has each of these fields only present in one direction. Polarised light can be created by the light source but it can also be created by polarising or “polaroid” sunglasses. Thus a non-directional light source such as light from the sun outside can be made into polarised light by passing it through a polaroid filter. As this cuts out all light oriented in the perpendicular direction, it reduces the intensity of the light passing through. That’s why polaroid sunglasses help on bright sunny days.
Once you have created polarised light by passing through a polaroid lens, it remains polarised with that orientation until something else happens. If you pass that light through a second polaroid lens, what happens depends on the orientation of the second polaroid. If the two polaroids are lined up, the light will pass straight-on through the second. If they are oriented at 90° to each other, there will be no light at all passing though the second lens. Try it. Hold two polaroid lenses (perhaps two pairs of sunglasses) up to the light and rotate the second pair relative to the first. You will see it go successively light and dark as the lenses line up.
What’s that got to do with sugar? Good question. Sugar solutions have the property that they can rotate the orientation of plane-polarised light. So if you have your two polaroid lenses at 90° and shine a light through (any light), you get no transmission through past the second lens. Put a sugar solution in between the lenses, you suddenly get light transmission again. To get no transmission, you would have to rotate the second polaroid. The angle you would have to do that by is a measure of how much the sugar solution rotated the plane polarised light.
A test carried out with a sample of sugar will make up a solution in a prescribed manner and establish the angle by which that solution rotates plane polarised light. From this is calculated a number called “pol”, which is expressed as a percentage. Pure sucrose has a pol of 100%.
Adding water reduces the pol (but as stated above doesnt actually reduce the amount of sucrose. Hydrolysis of sucrose to form glucose and fructose not only reduces the concentration of sucrose (and thus reduces the pol for that reason) but, because fructose rotates plane polarised light in the other direction to sucrose, hydrolysis actually reduces the pol by more than the amount of sucrose which has been hydrolysed.
Thus, if you have a cargo of bulk sugar, it is very important to sample it properly and representatively, and then measure the moisture content and the %pol. From these figures it is possible to calculate how much damage the sugar has suffered. Usually it isn’t as much as might be imagined from the cargo appearance.
As bulk sugar is hygroscopic, it should be ventilated according to the normal rules of seamanship. Having said that, condensation isn’t going to cause any genuine problems, so it doesn’t really matter what ventilation is applied.
Refined (white) sugar
This is usually carried in bags. There are a number of different grades of refined sugar, and I’m not going to go into the differences here. As a refined product, it is usually intended for direct human consumption. It tends to have a very low moisture content and will readily absorb moisture if exposed to the atmosphere.
Traditionally, the bags are made of woven white polypropylene, and they have a plastic film inner liner, which is stitched to the outer bag structure at each end. The polypropylene provides the mechanical strength and the film liner provides a moisture barrier. The moisture barrier is not a complete one – some moisture can get through the plastic film, and also moisture or even water can penetrate the film where the stitching has made holes in it. But the presence of the liner largely separates the very dry and hygroscopic sugar from the in-hold atmosphere. This means that for practical considerations, the sugar cargo in bagged form is not hygroscopic. Bagged refined sugar should not be ventilated. There are no circumstances in which it would be necessary to ventilate bagged sugar.
Indeed, there are circumstances where applying ventilation to bagged sugar can cause damage. Two mechanisms come to mind. The first is the classic “cargo sweat” situation. If inherently cold bagged sugar is loaded and carried to a warmer climate, and ventilation is applied, that can lead to condensation directly onto the bags and wetting of the bags. The sugar will remain cold throughout even a long voyage, and warm moist external air can cause substantial condensation onto the bags, particularly if the vessel has mechanical (i.e. fan-assisted) ventilation facilities.
The second mechanism is that ventilating with air of different temperature to the bags (warmer or cold) might be said to induce temperature fluctuations which could give rise to caking. As caking is one of the biggest problems experienced with sugar, it is important that nothing is done which might promote that caking.
Folklore suggests that sugar in lined bags can come into contact with splashes of water, but that isn’t recommended. Heavy condensation or wetting as a result of water ingress will have a tendency to penetrate the bags through the stitched ends. If that has happened, a region of syrupy sugar will be found at the end of such a bag when it is cut open. Most of the water will run off the lined bags, with only a small amount gradually seeping in through the stitches. This means that a stow of sugar in bags where there is water ingress from (say) a hatch cover leakage will suffer disproportionate numbers of damaged bags as a consequnce when compared to bags of rice which absorb much more of the water in the first few tiers affected. In way of a leakage, the water will find its way downwards leaving a pyramidal structure of wetted bags. The actual amount of water damaged sugar will be relatively small but that will be distributed amongst many bags.
Problems can also occur if bags are handled roughly, as the film liners can become torn. Likewise, if stevedores use hooks to lift the bags, the film liners will become compromised.
As I have said above however, caking is the most problematic issue with refined sugar, and that does not require damage to be bags or water ingress. Many articles have been written describing caking and what sort of inspections to carry out on caked bags identified after carriage. There are things you can do to investigate – like picking bags up and dropping them, cutting bags open to see whether the caking is present throughout or just the ends, and so on.
Sugar can cake very hard indeed. That is usually associated with the sugar not having been properly conditioned after refining. If sugar is bagged without conditioning, it can be too warm, too moist, or with moisture not equilibrated. These factors tend to give rise to hard caking by means of sugar being deposited in crystalline form between two adjacent granules, binding them together. If that process happens extensively in a bag, it can result in sugar which is wholly moulded to the shape the bag was compressed into in the stow without there being any straightforward means to return the sugar to its original free-flowing nature.
If a vessel is loading sugar which hasn’t been properly conditioned, there isn’t much they can do to prevent or reduce caking. It is a good idea to get an idea of the temperature of bagged sugar at the time of loading – but not using a spear thermometer!
Now we are Six
Absolutely nothing to do with A.A. Milne – sorry!
Iron ore fines
I outlined in last week’s post the three tests which have been a familiar part of the IMSBC Code for many years. In 2009 there were two vessels lost in India carrying iron ore fines – ASIAN FOREST and BLACK ROSE. In addition, there were numerous problems which arose during the loading of other vessels. At the time there was no schedule in the Code for iron ore fines as a Group A cargo. As a consequence, work was carried out resulting in new schedules and a new test. As I noted, the existing three tests came in for some criticism for not giving consistent results across a range of different types of iron ore fines. As a consequence, a new test was developed, which is now a part of Appendix 3 of the Code.
That test was based on the Proctor/Fagerberg test, and is referred to in the Code as the “Modified Proctor/Fagerberg test procedure for iron ore fines”. This test methodology makes it clear that it should only be used to test samples of iron ore fines.
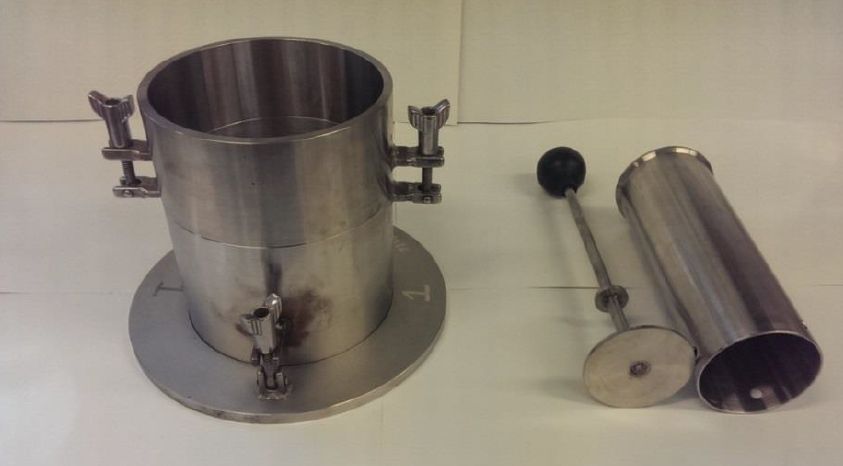
The standard equipment set was used but with a much lighter compaction hammer. This hammer weighs only 150g – less than half the mass of the standard hammer. The size of hammer required for the test was established by reference to work on the conditions experienced in a typical stow of iron ore fines, but it is very light indeed and imparts only small blows on the moulded sample. Even toffee hammers weigh more!
Incidentally, in Proctor’s original work on testing soils, the hammer weighed 2.5kg. Reducing the hammer size to 350g (standard Proctor/Fagerberg, referred to as the “C” hammer) and then 150g (modified P/F, referred to as the “D” hammer) provides substantially less energy input.
Unlike the standard Proctor Fagerberg test, the modified test for use with iron ore fines defines TML as the moisture required to produce 80% of saturation level. The justification for moving the critical point from 70% to 80% is touched upon in the wording of the test methodology in the Code. It says.
Optimum moisture content (OMC) is the moisture content corresponding to the maximum compaction (maximum dry density) under the specified compaction condition. … This test procedure was developed based on the finding that the degree of saturation corresponding to OMC of iron ore fines was 90 to 95%, while such degree of saturation of mineral concentrates was 70% to 75%.
IMSBC Code 2020 Edition, Appendix 3.
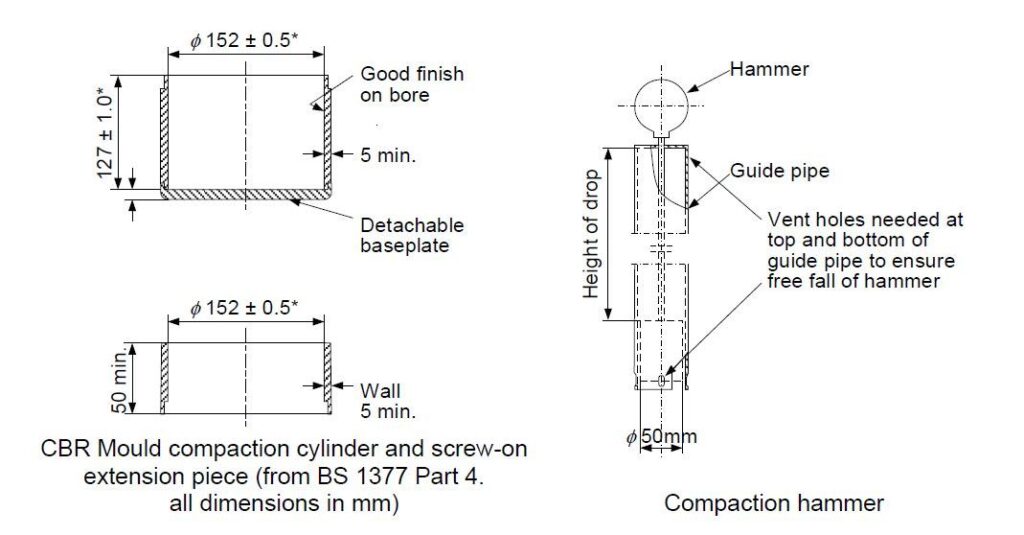
The concept of OMC is an important one. Imagine a sample with no water at all. Compaction using the hammer will result in a density which is governed by the intrinsic density of the solid material in the sample together with how well it packs into the mould (i.e. how much air remains after compaction). When water is added, initially the water will assist in lubricating particle to particle contact and will fill some of the voids – thus the density will increase because there is water in the gaps rather than air. Eventually, further additions of water will result in some free water within the sample mould because the voids are as filled as they can be. Because the density of water (1 kg/m3) is lower than the particle density of the ore being tested, adding further water serves to reduce the overall density. A mathematician would refer to this as a “turning point” in density, and the highest density happens at a moisture content known as OMC. OMC will depend on the compaction conditions – i.e. the weight of the hammer and to a lesser extent the shape and size of the mould.
Hence reasoning behind having a higher criterion for TML in iron ore fines. As the Code says, the iron ore fines samples studied had a higher saturation at OMC than was found when studing mineral concentrates using the Proctor/Fagerberg test. The aim is to set a TML which maintains a good safety margin (recall that for the flow table test and penetration test, TML is generally set at 90% of Flow Moisture Point). The reported fact that iron ore fines can typically attain a higher saturation than do concentrates means that the TML can be safely set to a slightly higher saturation level (80% rather than 70%). Well, that’s the theory.
As noted above, the modified Proctor/Fagerberg test for iron ore fines is only applicable to that cargo. This was the first time a TML test had been written into the IMSBC Code which related only to a specific cargo. Note that there is no prohibition on using any of the the original three tests on iron ore fines. The modified Proctor/Fagerberg test for iron ore fines is likely to give higher TML figures than would using any of the other three standard tests. It is odd that one of the driving forces beind the development of the modified test was that the existing three tests did not give consistent results. We now have a situation where a sample of iron ore fines can be tested by any of four methods, and these do not give similar results to each other.
Coal
The next method found in the Code is a modified Proctor/Fagerberg specifically for coal.
It is curious that the standard Proctor/Fagerberg method in the IMSBC Code contains the following statement
This method should not be used for coal or other porous materials.
IMBC Code 2020 Edition, Appendix 3, methodology for standard Proctor/Fagerberg
The way this provision is written is suggestive that the underlying reason the standard Proctor/Fagerberg method is unsuitable for coal samples is because coal is porous.
The modified method for coals uses a similar overall procedure to the standard Proctor method but the vessel has a larger diameter. The test method calls for a “hammer equivalent to the Proctor/Fagerberg D energy hammer” – more on this curiously worded requirement below. Critical moisture is set at 70% saturation, which is the level used in the standard test. If 70% of saturation cannot be attained in the test, the conclusion is that the cargo is not liable to liquefy. It is not clear to me how this addresses the implied difficulty in testing coal/porous materials implied by the wording of the standard method.
As noted above, the coal test method calls for a hammer equivalent to the D type energy hammer. That is the hammer developed for use with the iron ore fines test, which weighs 150g and has a foot diameter of 50mm. However, the image of the equipment for the coal test shows a different hammer. This has weight 337.5g (nearly as much as the standard C type hammer of 350g). However, the diameter of the foot is 75mm whereas the size of the standard D hammer is 50mm across. Thus the hammer shown for the coal method is heavier than the iron ore fines hammer but has a larger foot. It is equivalent because
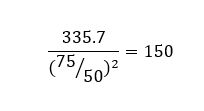
The most striking novel feature of the coal Proctor method is a procedure to create a reconstituted working sample where the coal contains particle sizes in excess of 25mm. Many commonly traded coal cargoes are sized up to 50mm, and so many real world coal cargoes will require the reconstitution procedure to be applied. The reconstitution process involves removing “oversize” (i.e. over 25mm in size) and then adding back calculated proportions of smaller particles from the working sample to replace the removed oversize fraction.
I mentioned in my earlier post on testing that a work-around to deal with oversize was in widespread use for nickel ore. The reconstitution process for coal samples is a part of the new modified Proctor/Fagerberg test for coals and is the first approved means of testing samples with particles larger than 25mm.
Many types of coal are not capable of liquefying and are thus not Group A cargoes. Very helpfully the new test contains criteria for determining if a coal is liable to liquefy.
I note that the new modified method for coal states that it requires a minimum of 170kg for a test to be carried out.
There are now three tests which can in theory be used for coal – the flow table test, the penetration test and the modified Proctor/Fagerberg for coal. The new coal test contains a number of elements which are not present in the flow test or penetration test – i.e. the ability to modify samples to allow for the presence of much larger particles and the existence of a diagnostic check in the test to determine whether a given coal is Group A or not.
Bauxite
The final method is specific to bauxite. The existence of this test came about as a consequence of the BULK JUPITER sinking and the work carried out which established and characterised dynamic separation as relevant to bauxite.
This modified Proctor/Fagerberg test uses the same larger diameter mould called for to test coal. The hammer is the lighter “D” hammer per the iron ore fines method in its 150g diameter 50mm form.
The bauxite method also contains a method for making a reconstituted working sample by removing oversize and substituting smaller material according to a formula/recipe.
The critical saturation level is set at 70% for some bauxites and at 80% for others. The decision between 70 and 80% saturation as the critical point is made by reference to the optimum moisture content – i.e. the highest saturation attained. This choice has at its technical base the same fundamental issue which is given as the rationale for applying a critical level of 80% in the modified test for iron ore fines as discussed above.
As I have discussed in an earlier article, bauxite is said not to undergo liquefaction in the normal sense, but rather to exhibit dynamic separation when overmoist. Dynamic separation is a different failure mechanism to liquefaction. However, the test introduced to ensure safe carriage and a suitable TML is largely the same as that applicable to iron ore fines.
This test is relatively new, and I have personally only seen it in action once. That particular bauxite formed large clay-like lumps with the addition of water and the 150g hammer was not capable of packing the ore into the Proctor mould except at very low moisture contents. Some bauxite types may be more suited to testing using other methods.
Concluding remarks
The recent proliferation of new cargo-specific versions of the Proctor/Fagerberg, together with the three more established standard methods presents a somewhat bewildering picture. Shippers involved in trades for coal, bauxite or iron ore fines will have the option of instructing a laboratory to carry out the cargo-specific test or one of the standard ones. Different commodities may involve the reconstitution procedures to accommodate substantial oversize. For commodities where no “new” cargo-specific test exists, the laboratory will have no established reconstitution procedure and will have to deal with oversize particles in an ad-hoc manner.
Clearly this is unsatisfactory. If reconstitution works for coal or for bauxite, it would be expected that a similar procedure might work for iron ore fines with oversize, or indeed nickel ore which tends to have oversize.
If it is acceptable to relax the Proctor/Fagerberg critical saturation percentage to 80% for iron ore fines and some bauxites, why is it not acceptable to apply 80% to any cargo which has an optimum moisture content (OMC) of over 90%?
If the studies showed that the new tests provide an appropriate TML for bauxite, iron ore fines and coal, where does that leave the “standard” methods in relation to these commodities? My understanding is that the modified Proctor tests introduced recently for these specific cargoes in a number of instances produce higher results for TML (i.e. the cargo can be shipped with higher moisture content). Perhaps the extra safety margin associated with the older tests was unneccesary.
It is possible to attempt to use the new tests for coal and bauxite and be directed by the test protocol to a conclusion that the subject cargo is not Group A. There are criteria in the schedules for iron ore fines, manganese ore fines and bauxite which set out technical parameters for what constitutes a Group A cargo of that mineral. There are no test procedures or criteria available for other materials to determine whether a given substance is Group A or not.
A number of materials mined and subject to relatively little processing contain a variety of minerals. For instance, an ore may contain nickel and also substantial amounts of iron. If an ore from Malaysia contains aluminium and also iron in oxide form, is it bauxite, or is it iron ore?
In my opinion, the proliferaton of multiple cargo-specific tests makes life very much more complicated for those involved and is not a helpful development. I believe it would have been preferable to revise the test methods in the Code to have either a single available test or a number of tests with the selection prescribed by the Code, rather than a “choice”. As always, however, we recommend adhering to the provisions of the Code wherever possible.
Testing, testing, 1, 2, 3
I wrote last week about liquefaction generally and tried to set the scene. Fundamental to safe carriage in the IMSBC Code is the concept of Transportable Moisture Limit (TML). It has been suggested that a single figure for a cargo does not properly reflect all possible behaviours, but in my experience the safety margin built into the Code is sufficient that if you apply the TML concept properly, the cargoes will carry without significant incident. Indeed, the idea of a TML has gained such traction recently in the maritime industry that I sometimes see figures for “TML” on declarations for agricultural cargoes which aren’t Group A at all – it is being erroneously used to indicate the maximum safe moisture level for avoiding cargo deterioration on board.
Returning to Group A cargoes, Section 8 of the Code tells you that there are test methods for determining TML, and the standard/recommended ones are to be found in Appendix 3 to the Code.
Unfortunately, Appendix 3 is where testing starts to get complicated. There are now no fewer than six different methods listed in the appendix. Three of these are variations on the Proctor Fagerberg test modified to be suitable for specific commodities and are relatively new additions to the Code.
Until recently, there were three methods listed in the Code, and those three will be the subject of this article. It has always been within the power of the various member Competent Authorities to approve alternative test methods, but as far as I am aware this has never been commonly encountered if at all. Furthermore, until relatively recently, the only method widely available internationally at commercial labs was the Flow Table Test.
The concept of TML was developed from another parameter, the Flow Moisture Point (FMP), and the original research into safe moisture levels and cargo testing was carried out on metal sulphide concentrates. Concentrates such as lead, zinc and copper have been shipped for many years and all tend to be Group A cargoes.
Flow Table
The flow table test measures FMP, which is defined as the moisture content at which a commodity starts to exhibit flow properties. The test involves inputting energy in a defined manner into the sample and monitoring the behaviour of the sample under those conditions.

Flow is defined for the flow table test when the cone of sample on the table spreads by a certain amount or slumps. If the sample doesn’t exhibit flow, water is added and the test repeated until it does. By this procedure, the flow moisture point is established by actually determining the moisture content of samples which have shown flow properties during the test along with those which do not.
One potential difficulty with the flow table test is that the methodology requires the operator to determine when a sample is in a flow state by observations during the test itself. The Code does not define exactly how, although there are descriptions of what to do.
Each test run involves a sample being loaded into the mould in a prescribed way. A spring-loaded device known as a tamper is used to squash the sample into the mould and provide a degree of compression/consolidation to start with. One problem with the flow table test which has largely now been solved is that the necessary calibrated spring-loaded tamper wasn’t available commercially.
Following removal of the metal mould shape, the resulting cone is measured prior to operating the table.

The table is then operated (up to) 50 times. Each turn of the handle lifts the table and allows it to drop 12.5mm. The method calls for the 50 drops to take two minutes.
After the table has been operated 50 times, the resulting material on the table is examined and measured. The IMSBC Code says measurements of the diameter are a useful diagnostic, and refers to a cone expansion of 3mm as being significant. Different laboratories have used this information in different ways. I can’t go into too much detail in this article but if full documentation of the test conditions is available from the laboratory, an expert can see how the stated result was obtained and what criteria were used.
The flow table is relatively inexpensive to buy, but it is important to ensure that a table meets the required design specification.
There are limitations associated with the flow table test. One is a consequence of the physical size of the mould into which the sample is placed on the table and relates to the maximum particle size.
The flow table is generally suitable for mineral concentrates or other fine material with a maximum grain size of 1 mm. It may also be applicable to materials with a maximum grain size up to 7 mm. It will not be suitable for materials coarser than this …
IMSBC Code Appendix 3
Widespread use of the flow table test for nickel ores having a proportion of much larger particles present has now been in place for a number of years, but the “fix” used has never been approved by IMO.
This fix works by considering nickel ore to be an assembly of larger particles embedded in a continuum made up of the more fine material. The flow table test criteria of 7mm is a workable limit for many nickel ore cargoes – removing the material above this size results in a testable working sample. The assumption made is that the liquefaction of the material as a whole (with the oversize) is solely governed by the behaviour of the sub-7mm fraction. The oversize is present in the cargo but is “along for the ride” without participating in the liquefaction. There is a further assumption which is that the moisture associated with the larger particles remains with them when sieved out, leaving the moisture associated with the finer material. Thus if flow table testing is carried out on the 7mm fraction alone, that provides a TML relevant to sieved samples. Provided the average moisture content is also tested on a sieved sample, the effect will be to ensure that the moisture level in the sub-7mm part of the cargo as a whole is below its TML, and hence the cargo can be assumed to be safe.
The obvious problem with this approach is that the moisture content of a sieved sample will tend to be different to the cargo as a whole. Most cargoes are bought and sold on terms which include specifying the cargo moisture levels. That isn’t for safety reasons but rather to act as a control for how much water is being bought rather than (here) nickel-bearing ore. Thus the moisture content entered onto the cargo declaration is not the same as the one used in the cargo sales contract. It is sometimes difficult to establish whether this has been done correctly.
Penetration Test
The penetration test was developed in Japan as an alternative to the flow table test, but it has certain similarities. This test is also repeated with additions of water until the sample exhibits flow properties. For this test, the energy input into the sample is in the form of a vertically oscillating platform onto which the sample is placed in a vessel. These vessels are much larger than the conical moulds used for the flow table test, and hence the penetration test can accept much larger particles.
The penetration test is generally suitable for mineral concentrates, similar materials and coals up to a top size of 25 mm.
IMSBC Code Appendix 3

The ability to accept samples containing much larger particles is one advantage of the penetration test. Another is that the definition of a flow state is given in terms of the distance penetrated by brass weights into and below the sample surface during the six minutes the vibrating table is run for on each test run. No subjective observation is required. The rate and amplitude of oscillation of the table are specified in the Code. In the video below a sample of iron ore fines can be seen to enter a flow state – signified by the brass weight penetrating the sample surface. Note that the video has been shortened from the original six minutes.
The flow table test and the penetration test have one fundamental thing in common. During the testing itself, each test run involves taking a working sample and subjecting it to the input of energy. This is meant to simulate the vibrations and motion on a ship, which is when liquefaction might take place. Water is added and the test runs repeated until a flow state is achieved, and thus the flow table and penetration test both create a flow state which the operator will see. In the flow table test, the sample will slump and expand during the test. On the penetration test rig, the metal bit will sink into the cargo sample, and other changes in the sample will be seen – perhaps water will come to the surface or the surface will become smooth.
Equally, if the sample is received in a state in which a flow state occurs on the first test, the operative will know immediately that the cargo as sampled will not be considered suitable for carriage.
The Code defines TML as 90% of the FMP for most materials. That 10% is a safety factor.
Proctor/Fagerberg Test
The third of the three long-standing tests is the Proctor/Fagerberg test. Until relatively recently this was not in widespread use in shipping, but it has always had its fans. This test works on a different principle to the flow table and penetration test. Here, the test runs involve packing the material of the cargo sample into a mould using a hammer. The weight of that hammer is specified by the method as 350g. The different test runs are not designed to result in liquefaction, and indeed no visual observations are required.
Liquefaction happens when the balance between solid particles, interstitial air and interstitial water is such that rearrangement of the assembly of particles when exposed to energy/vibration results in the interstitial water mediating particle to particle contact. The pressure of that water then supports the material above rather than particle to particle contact. This process was introduced with an illustrating diagram in my last article on liquefaction.
Proctor/Fagerberg replicates that compaction and rearrangement process using the hammer blows. As the density changes with increasing water, the test observations can be used to produce a curve from which the transportable moisture limit (TML) can be obtained.
The Proctor test does not produce an FMP and no observations of the cargo in a flow state will be possible during a test. The calculations required to process the raw data obtained during a test run, in order to plot a meaningful graph and establish a figure for the TML, are somewhat complex, and are a possible source of errors during testing. TML in this test is defined in terms of the moisture required to produce 70% saturation in the sample under compaction. Without the visual observations from the test itself there is no “sanity check” on the result obtained. That is one disadvantage of the Proctor/Fagerberg test.
The IMSBC Code sets out the calculations and invites the tester to plot the following graph.
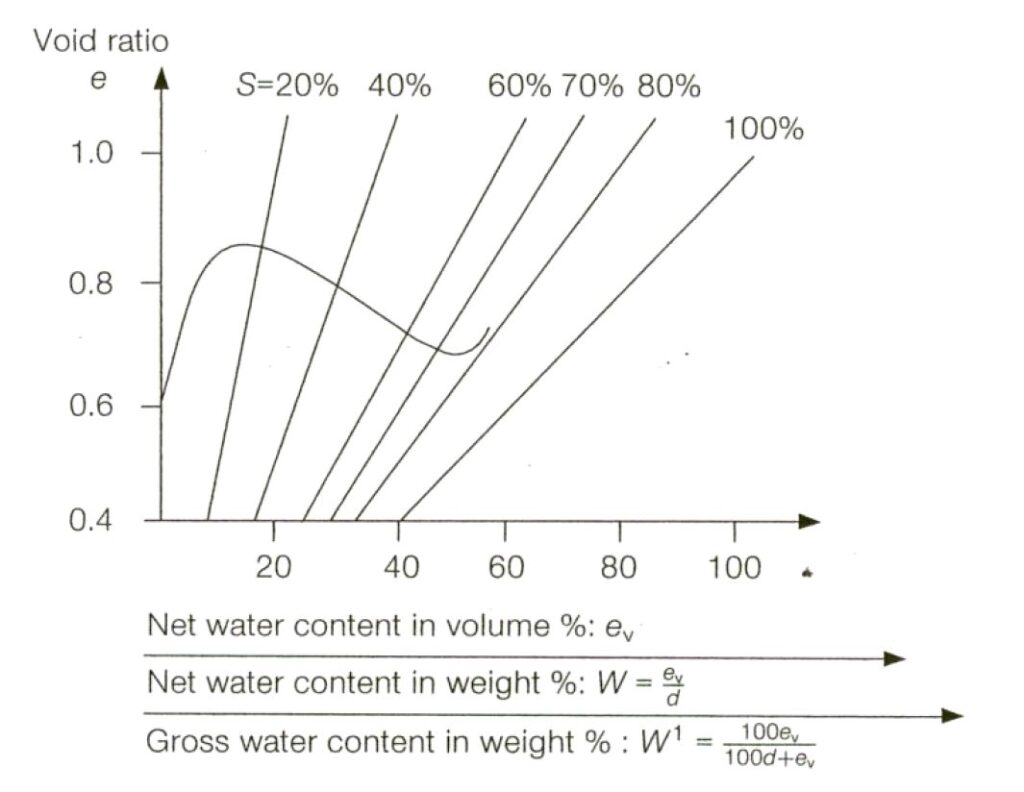
Fortunately the same data can be plotted in a (slightly) more intuitive way, as follows.
In this graph, the moisture content is plotted vs the degree of saturation that produces in the interstitial void space after compaction using the hammer.
Another disadvantage is again tolerance for large particles. The wording of the standard Proctor/Fagerberg method in the IMSBC Code indicates that it is suitable for materials with maximum particle size up to 5mm. The method goes on to imply that the method can be extended to work on substances having larger particles present but this needs to be investigated prior to deployment. Furthermore, the method states it is not suitable for coal cargoes.
For many years, those three methods were the only ones included in the IMSBC Code. For many mineral concentrates, the three methods would tend to give reliably similar results. Indeed, that was no coincidence. The parameters and TML criteria for the penetration test and the Proctor/Fagerberg test were in both cases selected in order to give similar results to the flow table test on some commonly-encountered mineral concentrate cargoes.
The problems started to become highlighted with the large-scale shipment of (first) nickel ore and then iron ore fines as Group A cargoes. Unlike mineral concentrates generally, these are not heavily processed commodities, and their behaviour when tested is not comparable with concentrate cargoes.
Nickel ore presents a number of challenges to a flow test. I have already mentioned the work-around in widespread use for large particles. Whlist this works, I don’t consider it particularly satisfactory. Unlike concentrates, nickel ore does not have a clearly defined transition from a dry state to a fluid liquefied state – you tend to find, if using the flow table test, that the cone expansion increases gradually over a wide range of moisture contents. Vagueness in the definition of flow state has resulted in large discrepancies in results between different laboratories when testing this material.
Iron ore fines entered the public gaze (the shipping public gaze, at least) a few years later. Iron ore fines cargoes of differnet origins can behave very differently when under test, and some cargoes also present the “oversize” problem but to a lesser degree than nickel ores.
There was considerable debate at IMO level regarding how to test iron ore fines for TML. The existing tests received some criticism for not giving consistent results for this commodity. As a result, when the IMSBC Code was revised to incorporate a schedule specific for iron ore fines, a new test was included at the same time. This test methodology was first published by IMO in late 2013 but was not fully incorporated as a mandatory part of the IMSBC Code until 2017.
Liquefaction – some basics
By now, most of those involved in shipping bulk cargoes know that some commodities can undergo liquefaction. Quite what that means is less well known, but it is very well publicised that it can result in ships sinking. Articles regularly appear setting out how many ships have been lost and the number of people who have lost their lives when their vessels have got into difficulties during carriage.
There is much less information available on the “nearly” events, or even the “we worked hard to avoid” events. I have personally been involved in a substantial number of both of these. Timely advice and/or intervention can save ships and lives. I’m pleased to be able to say that no vessel I have been involved with has subsequently been lost. Suffice it to say that the cost to the industry of problems arising from liquefaction generally is very high.
IMO categorises bulk cargoes which can liquefy as being in cargo Group A.
To comply with the regulations, the moisture content of a Group A cargo needs to be below the transportable moisture limit (TML).
Concentrates or other cargoes which may liquefy shall only be accepted for loading when the actual moisture content of the cargo is less than its TML
IMSBC Code 2020 Edition paragraph 7.3.1.1
What is TML and how is it measured? In a classic example of circular definition, the Code tells you that TML is
Transportable moisture limit (TML) of a cargo which may liquefy means the maximum moisture content of the cargo which is considered safe for carriage in ships …
IMSBC Code 2020 Edition section 1.7
A later post will discuss the tests, as these and how they work is a whole topic in itself.
Liquefaction is a bulk property. One common misconception is that liquefaction involves cargoes becoming a liquid, and indeed the IMSBC Code uses this word to describe a flow state. Many liquefied cargoes however do not behave like liquids. “Fluid behaviour” is perhaps a better term to use.
As a physicist, I will introduce the concept of a phase change, as I believe the analogy is helpful. To a physicist, a change of phase involves a material changing from a state in which it has certain properties to a state where it behaves differently. An obvious example is melting a solid such as ice. Whilst the ice is frozen, its behaviour is like other solids. Melted, it becomes water which can be poured, sloshed, and so on but it is chemically still H2O. The phase change we refer to as melting involves the input of energy. So does liquefaction. A solid bulk Group A cargo can may enter a fluid state if conditions are within certain limits and with the input of energy.
A very widely viewed video shows a wheelbarrow full of soil undergoing liquefaction when energy in the form of vibration over a set of cobblestones is input.
Back in 2009, the year ASIAN FOREST and BLACK ROSE sank in India, I created this diagram.
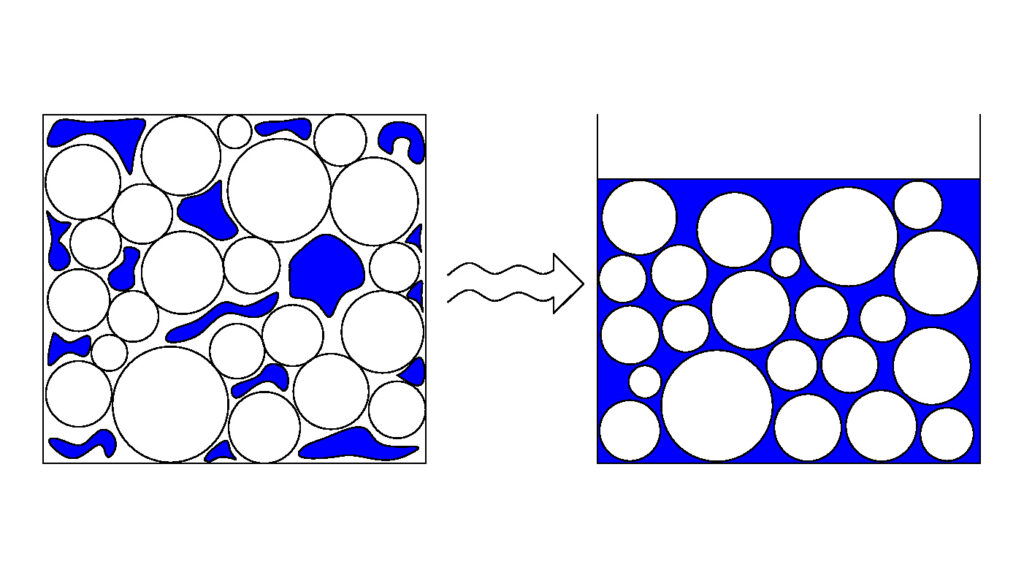
Facsimiles of this illustration exist, even variants where the particles are not circles/spheres. All the attempts to “improve” on my illustration/concept focus on aspects of it which didn’t really need to be updated/modified.
In this example, the assembly of particles is shown to be a collection of spheres (circles in cross-section). That wasn’t chosen because it matches the particles in a cargo of iron ore or nickel ore, but rather because it allows the phenomenon I am illustrating to be clearly seen. The diagram shows a phase change. On the left, the solid particles are loosely packed. There are lots of spaces between them. In those spaces can be found the moisture associated with the cargo (the blue areas). The particles are in contact with each other and the water (blue) is simply present. It doesn’t do anything.
Follow the wavy arrow to the right hand side; there to represent the input of energy in the form of vibration during a sea passage. In the right hand image, you will find exactly the same circles/spheres. Here however the whole assembly occupies a smaller volume than did the left hand image. Thus the input of energy has resulted in consolidation of the cargo into a smaller volume. Further, the image shows blue water throughout the interstitial spaces.
The point the image is illustrating is that the sequence of events involves consolidation/compaction as a consequence of the input of energy. The same volume of solid particles is now associated with less interstitial volume and, if there is enough moisture present, that might result in the water filling the voids on the right hand side when it did not on the left. Liquefaction. The particles on the right are separated by water and it is the water which governs the bulk properties. Pore water pressure is the name given to the pressure associated with the water in the spaces, and in a liquefied stow it is the pore water pressure which supports the cargo above. Water, being a liquid, is nearly incompressible – the volume of water stays the same at all times, it is the volume of the interstitial pores which changes.
The Proctor/Fagerberg test works on this principle – the test is designed to measure the amount of water expressed as a volume percentage of the available space (void/interstitial). Thus the image on the right of my diagram would be 100% saturated in Proctor Fagerberg terms. The energy input in the Proctor test comes from a slide hammer pounding the surface of the sample. I will write about the tests and how they work in a later article.
Many of the provisions in the IMSBC Code (or BC Code as it was a number of years ago) arise from studies carried out on samples of metal concentrates. It is worth remembering this when consulting the Code even today. Concentrates are a semi-manufactured intermediate product and tend to be very consistent in terms of particle sizing. The provisions in the Code allow for TML certification to be up to six months prior to the voyage. These make sense if we are dealing with a cargo of concentrates from a process which churns out parcels of that commodity which are very much the same in terms of flow properties from month to month, even year to year. The same provision makes no sense whatsoever when dealing with shipment of nickel ore or iron ore fines; these receive little processing prior to shipment and the flow properties depend on the geological conditions in the mine.
There are a number of phenomena reported regularly when vessels are affected by liquefaction. As I have said above, the cargoes tend not to behave like liquids, but rather very dense fluids. The stow may flatten out – an untrimmed/peaked stow may turn into a wholly flat surface. There may be water expressed to the surface of the stow. Look at my diagram above – on the right hand image there wasn’t enough room for the inherent moisture and some of it ended up on top of the cargo. That water is simply a liquid and will be free to form a free surface. A free surface of water will affect the stabilty of the vessel but often the amount of water actually on the surface isn’t large, even though it may look spectacular when in motion.

When many cargoes liquefy, the whole stow, or large parts of it, can become mobile. That is a result of the particle to particle contact being prevented by the interstitial water. There are often reports of the vessel taking on a list. This tends not to be an angle of loll, but rather a result of the cargo moving. What can happen is that when the cargo becomes mobile when in a fluid state, it moves to one side with the natural roll of the vessel. It then doesn’t return to centre when the vessel rolls back. Gradually, over a number of roll periods, the cargo takes up position biased towards one side of the hold, resulting in the list. This is of course very dangerous as eventually that list may become large enough for down-flooding with sea water to occur. This is one example of liquefied cargoes not behaving as liquids as it is the asymmetric behaviour of the cargo which results in the list developing.
The case of bauxite as highlighted by the tragic loss of the BULK JUPITER and the lives of 18 of her crew members is interesting. Work followed which resulted in new provisions in the IMSBC Code for Group A bauxite cargoes. It has been strongly argued that bauxite does not undergo liquefaction, and a new term “dynamic separation” has been coined to describe its behaviour. This BIMCO article has a clear description of the difference between dynamic separation and liquefaction. In dynamic separation, the cargo splits into two phases. A lower phase containing the cargo solids is initially immobile despite being saturated with water. It is not in a liquefied state. There is however a volume of free water expressed to the surface (which may be a slurry containing some very fine particles of solid cargo). That water phase is mobile and the risk to the ship’s stability comes from the free surface effect of the water. Only when the vessel’s stability has been compromised by the free surface effect to produce a substantial list might the lower solid phase move.
The new bauxite fines schedule has an interesting provision.
An atypical motion of the ship (wobbling) may also be indicative of cargo instability and the master shall consider appropriate action.
IMSBC Code 2020 Edition
I have heard presentations from members of the bauxite working group where this “wobble” was stressed as one of the clear “red flag” moments a vessel should be watching out for when carrying bauxite fines. A video is often shown which was produced by AMSA to illustrate the behaviour of vessels encountering dynamic separation. I would encourage the reader to watch it carefully.
It is important to note that the video does not show anything liquefying. The motion seen by the model in the video is governed by its buoyancy and the motion of the steel balls in the tracks. Those tracks are shaped to produce a harmonic motion rather like a pendulum. Having two or more balls on the tracks at the same time produces a form of coupled motion. Coupled harmonic oscillators can exhibit extremely unpredictable behaviour. The well-known Rotts Pendulum is an example – this couples two pendulum rods with different lengths and weights. The resulting behaviour can swap back and forth between wobbles and seemingly random modes.
The AMSA video is simply showing coupled harmonic oscillators on a floating system. As an illustration of the consequences of dynamic separation this is appropriate because it is a free fluid phase (the expressed water) which is compromising the stability. This is not the same mechanism which gives rise to the asymmetric behaviour I have described above for cargoes undergoing liquefaction.
For a vessel at a port loading a cargo, the distinction between liquefaction and dynamic separation isn’t obviously significant. Both are behaviours exhibited by Group A cargoes. Both require a TML to be certified and require the moisture content of the cargo to be below the TML for safe carriage. Many natural unprocessed ores contain a mixture of different minerals. Thus the distinction between nickel ore, iron ore and bauxite may depend more on the intended processing and use than the actual make-up of the ore itself.
Vessels carrying Group A cargoes have successfully reached discharge port with mobile cargo in one or more holds. It is however a huge risk. Similarly, carriage of cargo which is overmoist does not necessarily result in either liquefaction or indeed in the vessel getting into difficulties.
The reverse however is clear. To the best of my knowledge, no vessel has loaded cargo which meets the criteria of the IMSBC Code (i.e. with average moisture properly below a reliably tested TML, properly trimmed) and has subsequently had difficulties with cargo liquefaction unless there has been actual water ingress into the holds.
The message then remains the same as it always has. If you wish to carry a Group A cargo safely (and you should), the IMSBC Code should be followed. That means proper sampling being carried out prior to loading, and those samples being reliably tested for moisture and a TML established by a laboratory properly equipped and experienced in carrying out the tests in question. In my opinon there is no substitute for this which can guarantee the same degree of safety.
Poynting and Birmingham
During a recent visit to Birmingham University, I spotted a blue plaque commemorating physicist John Henry Poynting.

Zooming in on the blue plaque, we see that Dr Poynting is celebrated on this building for having measured the mass of the earth.

You can’t put the earth on a set of scales, but you can use precision balances to measure the gravitational effect of objects on each other, and use that to calculate the mean density of the earth. From that, you can calculate the mass. Poynting spent 12 years at Cambridge and then at Birmingham carrying out these experiments. Although that is what the blue plaque records, it is some of his other work which resulted in his name being being well known to physicists.
Dr Poynting was the first Professor of Physics at Birmingam University, when it was formed under that name in 1900. He had been heavily involved with the university’s formation, having been a professor at the Mason Science College, effectively a predecessor organisation before Queen Victoria issued the Royal Charter to Birmingham University in March 1900.
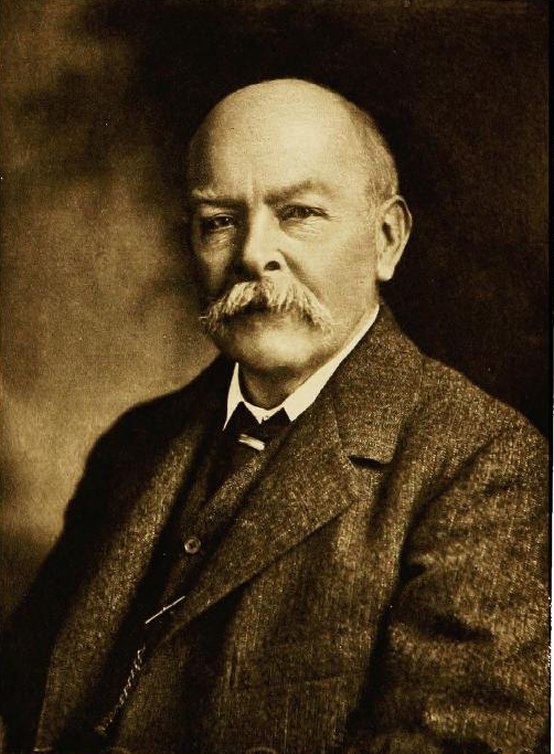
Poynting had worked at the Cavendish Laboratory, Cambridge under James Clerk Maxwell, one of my scientific heroes. After Maxwell died, Poynting was one of a group of physicists who collaborated to rework Maxwell’s equations of electromagnetism into their familiar form.
Professor Poynting developed an expression for an entity familar to all physicists which is known as the Poynting Vector. This, as its name suggests, is mathematically a vector (i.e. it has a direction as well as a size). It is the magnitude and direction of the energy flow in an electromagnetic field or electromagnetic wave. Electromagnetic waves include light of all colours and wavelengths, radio waves, infra-red light, ultra-violet light, X-rays, and some forms of radioactivity.
S = E x H
Poynting Vector S in terms of electric field E and magnetic field strength H. “x” denotes the vector “cross” product
In an EM wave, the electric field and magnetic field are at right angles to each other and the energy flow (the Poynting Vector) is at right angles to both of these. Poynting established also that electromagnetic waves, including light, exert a pressure on a surface they hit, and the greatest pressure is to be found in the direction of the Poynting Vector.
Poynting’s life and work brought him into contact with many of the other most famous physicists of his time. I have mentioned Maxwell above. After leaving Cambridge, Poynting spent some time teaching at what would become Manchester University. One of his students was JJ Thomson, later famous for discovering the electron. One of my own most inspirational teachers encouraged his classes to refer to Thomson as JJT as if we all knew him personally!
Dr Poynting and JJT collaborated on a physics text book which was still in print half a century later. This book was rather modestly entitled “A Text Book of Physics”, but was in very widespread use in Physics teaching.

Although non-Physicists may not have heard of Professor Poynting, his work on electromagnetism and EM waves amongst other subjects was a hugely important contribution to knowledge in the latter part of the 19th Century and the early part of the 20th.
Mind the Gas
Fumigation of ships and cargoes has for many years been a source of problems to ship and cargo owners. Recently the UK MAIB issued a preliminary report into a serious incident on the vessel THORCO ANGELA, which happened in Liverpool in October 2021.
Insects are a fact of life. The Royal Entomological Society estimates that there are around 1.4 billion insects per each human alive on earth. The total mass of insects on earth is, they estimate, around 70 times the mass of all of the humans.
Hardly surprising then that agricultural cargoes (grains, oilseeds, animal feeds, etc) are at risk of becoming infested. Nobody wants that, and to avoid infestation cargoes must be treated every few months.
Insecticides can be sprayed onto a surface or cargo (“contact insecticides”) or can be applied as a gas (fumigants). Treating a large bulk with contact insecticide would require it to be sprayed (for instance) onto cargo on a conveyor. Using a fumigant is often more convenient as the gas can penetrate a large bulk.
The disadvantage is that it takes time for fumigant gas to diffuse throughout a large bulk. If in that time the gas starts to leak out, that’s bad for two reasons. Firstly, the fumigation may not be sufficiently effective (because the gas wasn’t present throughout the stow for long enough). Secondly, and much more important for safety reasons, if the gas isn’t staying the holds, it is going somewhere else. Fumigants are used because they kill insects. They can also kill humans or at the very least make them very ill indeed.
Most fumigation on ships these days uses a fumigant gas known as phosphine, chemically PH3. With very few exceptions this is deployed on ships by introducing solid preparations (tablets or pellets etc) into the hold. These solid materials contain aluminium or magnesium phosphide. These chemicals slowly react with atmospheric moisture in the hold and release the phosphine gas itself.
Phosphine is very dangerous. The recommended limit for spaces occupied by humans (NIOSH time weighted average) is 0.3ppm. But it isn’t just the toxicity. Phosphine is highly flammable in air, and in the real world, impurities commonly found in it can make it spontaneously explosive. Hatch covers can and have been blown into the air by phosphine explosions. The solid materials themselves can cause fires if they become wetted.
Fumigation on ships is, for these reasons, subject to IMO rules, recommendations and regulations. The IMO publication “Recommendations on the Safe Use of Pesticides on Ships Applicable to the Fumigation of Cargo Holds” has quite an unwieldy title, but contains guidance which should always be followed to ensure safety of life and vessel. This publication can be found as IMO Circular 1264, but it is also bundled as part of the IMSBC Code.
One part of the safety requirements is appropriate signage to indicate when fumigants are in use.

Standard shipboard gas meters are not designed to detect phosphine gas (although they have a sensor which may be referred to as a “toxic gas” sensor, they won’t help in keeping personnel safe). Specialist electronic meters exist, but they are quite expensive and no use for other tasks. Inexpensive glass detector tubes (made by Draeger or Gastec or similar) can be used but each tube can only be used once so you would get through a lot of such tubes in any particular practical deployment.
Lots of articles have set out the provisions to be found in IMO Circular 1264. I’m not going to do that here but I am going to discuss a point which came to the fore during the lockdowns applied as part of the worldwide response to the Coronavirus pandemic.
In the Circular, the following paragraph can be found.
Since fumigant gases are poisonous to humans and require special equipment and skills in application, they should be used by specialists and not by the ship’s crew.
IMO Circ. 1264. Para 3.1.2.2
Recommendations on the Safe Use of Pesticides on Ships Applicable to the Fumigation of Cargo Holds.
This provision is very clearly worded. The pandemic however caused all sorts of problems with many aspects of world trade. One particular trade customarily posted a trained fumigation operative to sail with a vessel as a supercargo. One of his duties was to monitor gas levels in the holds and add more solid phosphide preparation into the holds to “top up” the gas levels. I presume (or hope!) he would also carry out checks to establish where the gas was going, and to ensure that wasn’t going to cause problems.
With the Covid pandemic, this was no longer allowed. Getting personnel on and off ships became virtually impossible in many instances, and so a supercargo riding with the vessel was no longer viable.
The shipping world is full of “make do and adapt” attitudes – admirably so in many instances, but not here in my opinion. Initially, crews were supplied with the solid materials and asked to top up when required. No doubt this was done without incident on a number of occasions but it does violate the IMO Recommendations. As consultants we did not recommend carrying this out.
A second level of “work-around” was developed in which fumigation companies provided specific training to a designated crew member who thus became a “specialist”.
The organisations which were involved in this trade were relatively sophisticated and no doubt took this training seriously. However, once a precedent is created in which crew members are handling fumigants and fumigation materials, there becomes a possiblity that problems may arise if untrained crew members become involved.
Turning to the THORCO ANGELA incident (note, Sheard Scientific were not involved in investigating this), the MAIB report indicates that she loaded sweet potato in Rizhao, China. MAIB concluded that trained fumigators had not been allowed on the vessel and nor were any crew members supplied with sufficient training or equipment.
MAIB also conclude that the deployment of the solid phosphide material was done in such a way that the fumigant phosphine did not adequately “volatilise and disperse”. If the solid phosphide materials still contain substantial unreacted phosphide at the end of a voyage, they will still have the propensity to give off fresh phosphine gas.
One of the stevedores involved in discharging the cargo from THORCO ANGELA became unwell and was taken to hospital suffering with nausea, loss of balance, and nerve damage to his hand. MAIB note that Liverpool port had not been informed that the cargo was under fumigation. Thus the stevedores had no advance warning that there might be a problem.
This was undoubtedly a very serious incident, and it could easily have been very much worse. The takeaway lesson for those involved in carriage of cargo under fumigation should be to ensure that where alternative procedures are introduced (perhaps because there was no alternative), all effort should be made by all parties to make those alternative procedures as safe as the original ones. If ship’s crew are going to carry out the tasks which should be carried out by specially trained personnel, that can only take place if the crew members in question genuinely have received sufficient training.
A trip to Whitby – Dracula, Captain Cook and the Scoresbys

A younger member of the Sheard household is studying literature (and fancies herself a bit of a Goth), so we had a trip to Whitby in North Yorkshire to see what we could discover about Bram Stoker’s inspiration for Dracula.
Vampires aside, Whitby of course has a proud maritime history. Captain James Cook was born and lived nearby and explored huge previously uncharted parts of the world.

The focus of our trip however was Dracula, written by Bram Stoker, a frequent visitor to Whitby.

In the story, Dracula arrived at Whitby by sea and some of the novel is set in the graveyard of St Mary’s Church, near the Abbey.
Wandering around the churchyard, we didn’t find any vampires but we did find numerous Master Mariners. Many of the graves are actually memorial stones to those lost at sea.


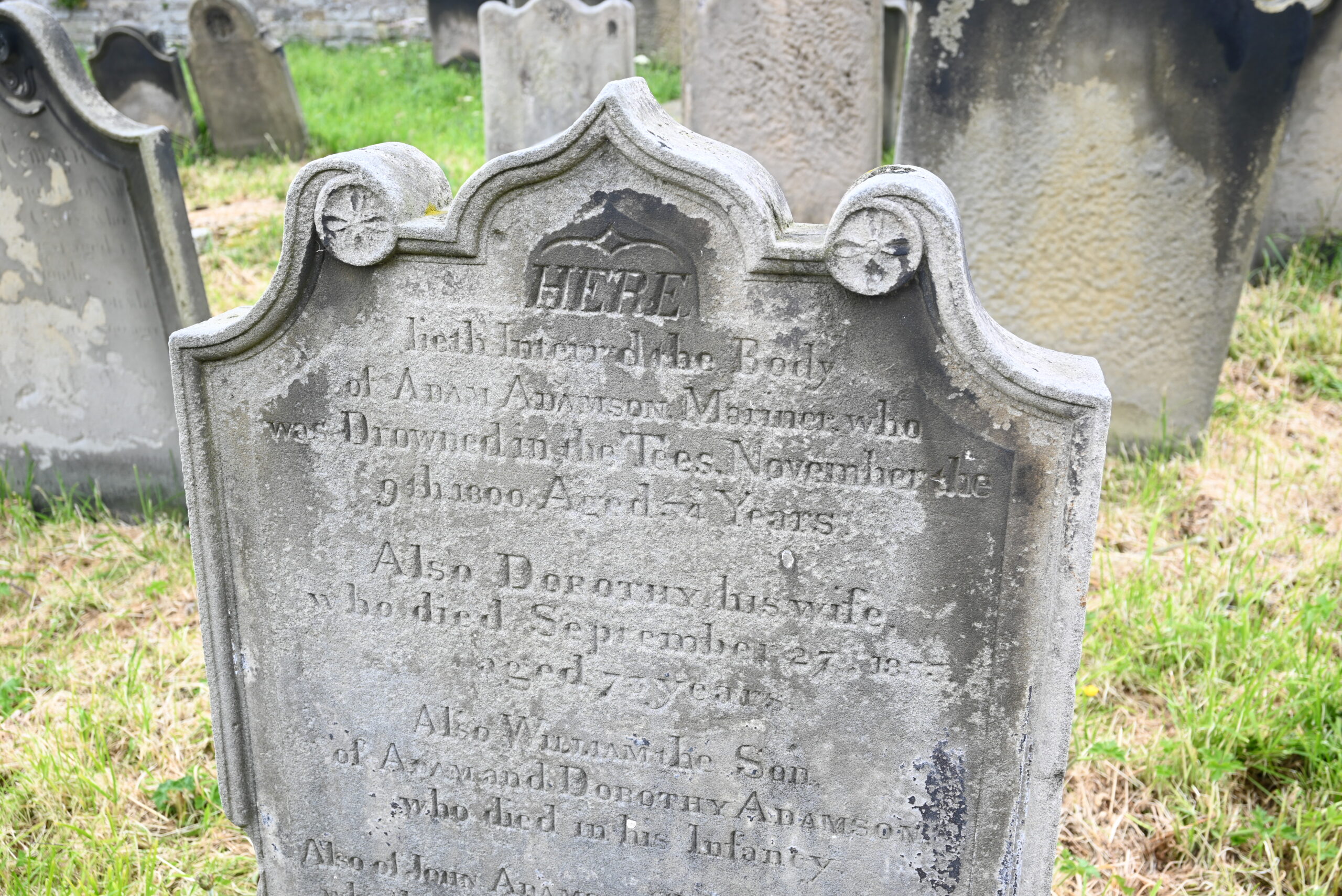
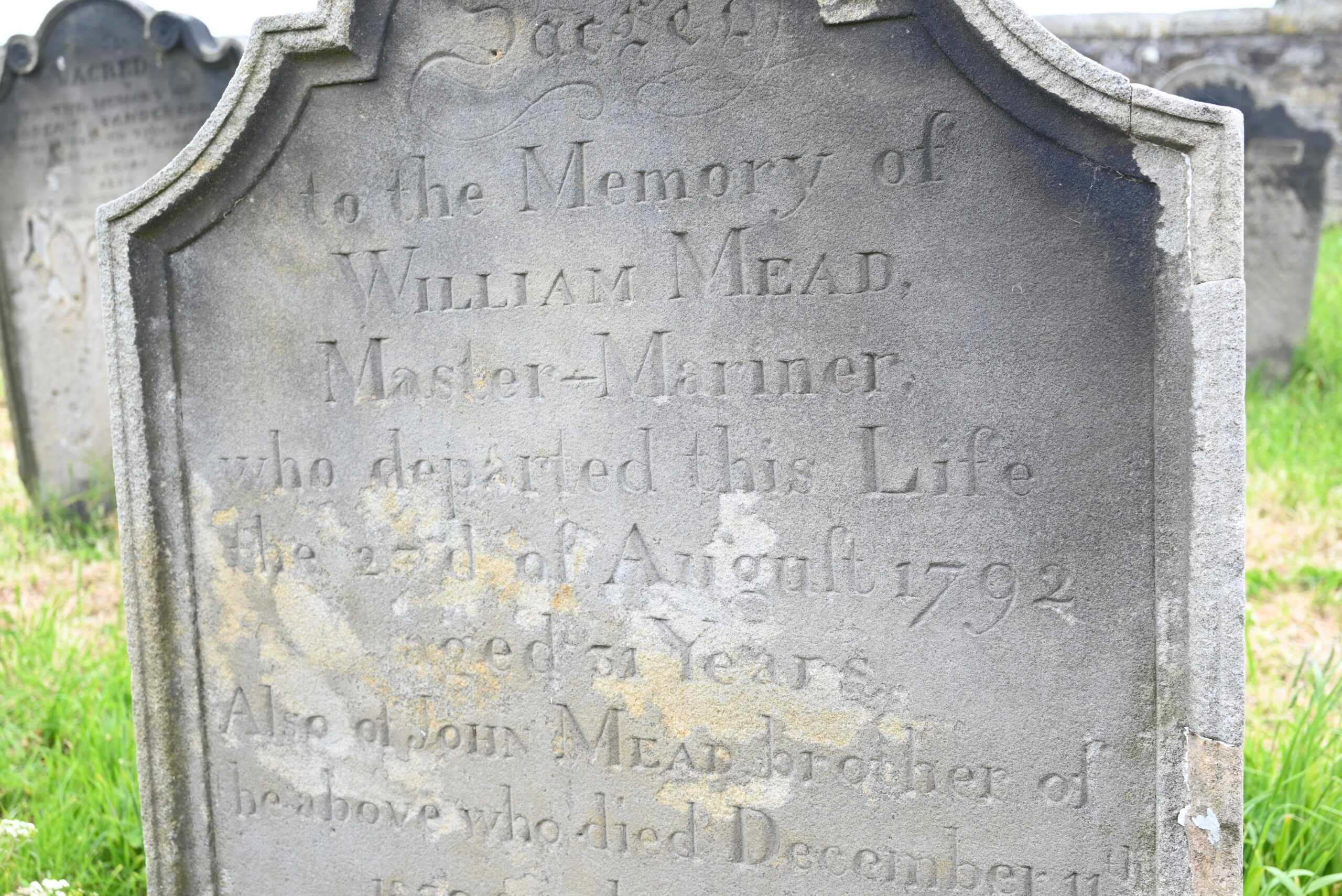
Nearby in the churchyard, however, we located something of significant interest historically and scientifically – the grave of William Scoresby.


William Scoresby made his fortune in Arctic whaling. He is often credited as the inventor of the ship’s crows nest. Elevated lookouts on ships may have existed prior to Mr Scoresby, but he apparently did originate the barrel crows nest.
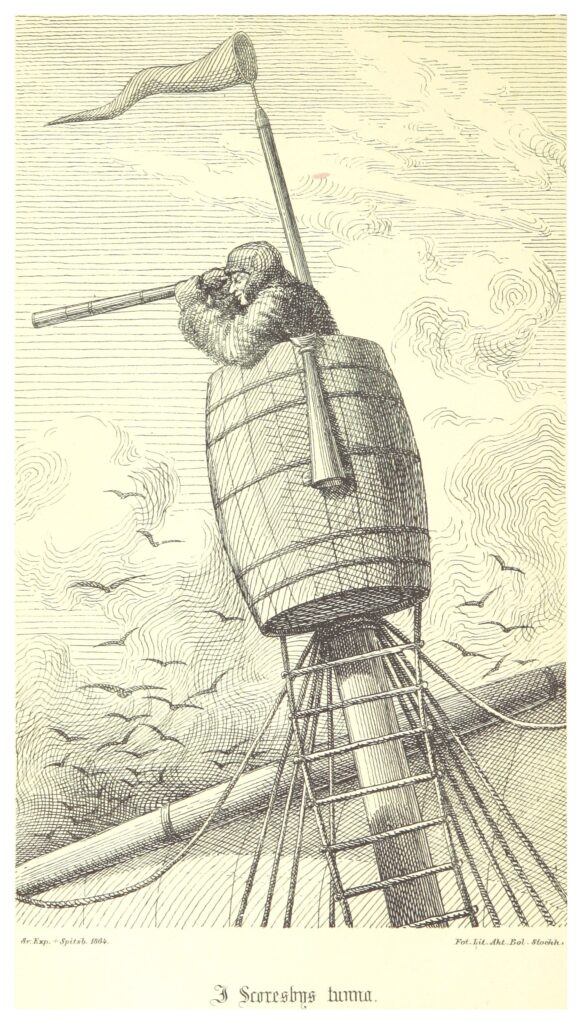
In the church itself is a chair known as the Scoresby Chair, which was carved from the remains of the vessel ROYAL CHARTER, on which William’s son, Rev William Scoresby carried out scientific experiments.







Rev William Scoresby spent many years working on the problem of compass deviation on ships incorporating iron as part of their structure. His views brought him into conflict with the Astronomer Royal, Sir George Biddell Airy with such vehemence that the issue became known as the Airy-Scoresby Controversy.
The problem is that iron in a ship’s structure can become magnetised and interfere with the operation of a navigation compass. Numerous ships were lost as a consequence.
Airy proposed a solution in which the ship would be “swung” through the points of the compass and the behaviour of the navigation compass checked at each orientation. Iron masses were then added in the vicinity of the compass to neutralise empirically the effect of the iron ship structure on the compass readings.
Scoresby believed this approach was so wrong as to be dangerous, and said so during a speech he gave to the British Association for the Advancement of Science in Liverpool. He believed the magnetic effect of the iron in a ship’s structure could suddenly and unpredictably change as a consequence of the vibrations set up by her motion in a seaway.
To investigate this, Rev Scoresby sailed around the world via Australia on ROYAL CHARTER in 1856 and took a large number of compass readings during the voyage. He aimed to demonstrate his theory of shifting magnetic influence by means of these observations.
Rev Scoresby died in 1857, and ROYAL CHARTER sank in 1859. The outcome of the controversy was that Airy’s system of compensating massess was retained, but one of Scoresby’s innovations, locating a “pole compass” on a mast and away from the ship’s iron structure, was incorporated to improve maritime safety.
A small part of ROYAL CHARTER lives on in the Scoresby chair and if you visit Whitby, I suggest you take a look.

Biotechnology, GMOs and Gene Editing – Part 1 – Introduction
I am sometimes asked to assist in matters which involve detection of genetically modified organisms (GMO). This is very important in some trades as many countries have lists of approved cultivars and stricly only those are permitted to be present. Organic trade is also highly sensitive to contamination by anything which is not appropriately certified as organic.
The technology to detect the presence of modified organisms, and identify any which are present, is very advanced but you need to have an understanding of what the tests are, how they work, and what they look for in order to understand what results do or do not mean. As with many things, the first step in testing is obtaining reliable samples. If the samples are not properly representative of the goods in question, the testing results will not be useful.

Anything related to genetics generally has its basis in DNA. DNA stands for deoxyribosenucleic acid and it contains the information required to make the proteins out of which life is built. The double-helix structure of DNA is like a ladder which has been twisted. The backbone structure (made up of deoxyribose sugars) forms the sides of the ladder and the rungs are nucleic acid pairs. Each chain of deoxyribose sugars has a sequence of nucleic acids, each of which is hydrogen bonded to a complementary nucleic acid on the other half of the ladder.
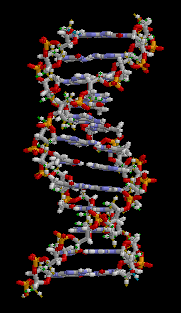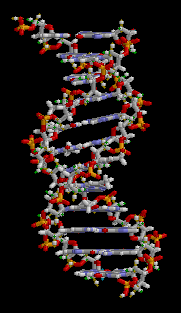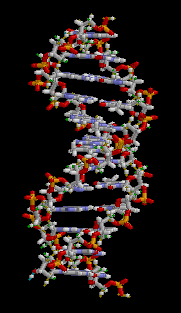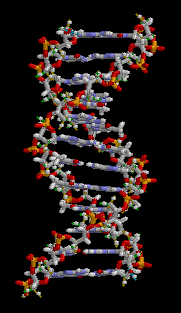
There are four nucleic acids, which are adenine, thymine, cytosine and guanine (denoted A, T, C and G). They are known as bases. A always pairs with T, C always pairs with G. Thus if you were to pull apart the two strands of DNA and throw one strand away, the nucleic acid sequence for the missing strand can be recreated by matching to the remaining strand. This property of DNA is what makes it possible for it to be reproduced. Thus, when DNA is duplicated in a cell, this happens by the DNA splitting into two strands, on each of which a replacement complemetary strand is created. Without this property, life as we know it would not be possible.
The genetic information in DNA consists of the bases and the order in which they are found. DNA is found in the nucleus of the cells and the information in it is used to make proteins and enzymes etc. The first stage in this process is that the DNA is transcribed to RNA. A part of the DNA is separated into two strands, and one strand is used to make an almost copy of part of the DNA sequence. RNA stands for ribose nucleic acid. This is very similar to DNA except the sugar backbone is ribose itself rather than deoxyribose. RNA consists of a sequence of bases which are complimentary to the DNA it has been transcribed from, but RNA does not use thymine; instead the RNA has uracil (U). Thus a DNA sequence made up of bases such as GATTACA would be transcribed into RNA as CTUUTGT.
The RNA transcription (referred to as messenger RNA or mRNA) is then transported out of the nucleus to a different part of the cell known as a ribosome. There the genetic information is translated into the product for which it codes. To do this, individual amino acids are added to a protein chain which gradually builds up to be the product. The bases on the mRNA are taken three at a time, and combinations of bases code for different amino acids. A group of three bases is known as a codon. The table below contains the so-called genetic code – i.e. what amino acid a given codon/combination of three bases codes for.
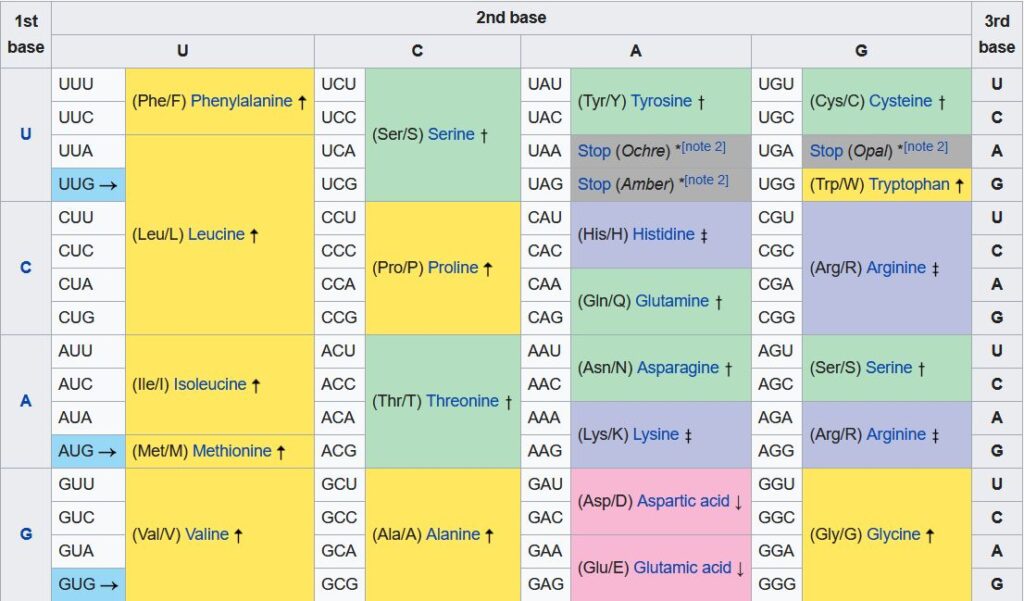
Thus a triple which reads GCC would result in alanine being added to the protein chain, and so on. The order of the bases in the codon matters, and also it is important to note that if the “reading frame” is shifted by a base pair, the product is different. Thus CUUAGUGGU codes for leucine followed by serine and then glycine, but if a base is removed from the beginning of the sequence, the resulting UUAGUG… codes for leucine followed by valine.
This then is known as the central dogma of molecular biology, stated in very simplified form as
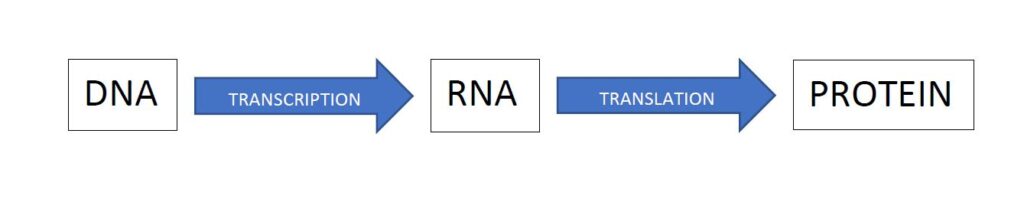
The arrows designate information flow. All life on earth relies on this.
As an aside, there are occasions when information flows from RNA to DNA. So-called retroviruses such as HIV contain RNA. Part of that RNA codes for a special enzyme known as “reverse transcriptase”. This converts the viral RNA to DNA which is then inserted into the target cell DNA for replication by the host cell when it replicates. Sneaky! Coronaviruses such as COVID-19 are also RNA viruses but they code for enzymes which replicate the RNA to RNA, they don’t usually get inserted into the host DNA.
GMOs and gene editing techniques all rely on modifying the DNA sequences to produce a desired product. That product will tend to involve the plant (usually) cells producing proteins the native plant type cannot or does not.
Dominoes

The inter-related nature of world trade is often mentioned when things go wrong. For instance when in 2021 EVER GIVEN became wedged in the Suez Canal, there were significant knock-on effects felt worldwide.
The current conflict in the Ukraine is another example. A substantial amount of grain including sunflower seeds, maize and wheat comes out of Black Sea ports. Currently a number of vessels are stranded in Ukraine ports unable to leave and caught in the conflict. The disruption to the world food supply is becoming widely known.

As an example of the consequences, take a look at the supermarket shelves for cooking oil next time you shop. It appears that sunflower oil is becoming difficult to get hold of. Instead the shelves might carry non-specific vegetable oil which may contain rapeseed oil. Fields of yellow oilseed rape plants used to be a common sight in the UK, but in recent years less acreage has been devoted to this crop because of a ban on neonicotinoid pesticides implicated in causing problems for bees. Will we start to increase the amount of rapeseed planting? Maybe.
Another alternative is palm oils. The environmental implications of large palm plantations are frequently stated and are widely accepted, but the plant does produce a number of oils which have multiple uses. However, the Indonesian government has recently announced a ban on palm oil exports. This is reportedly being put in place to ensure that sufficient supplies of oil are available for domestic use in Indonesia.
One to watch out for may be coal. The industrial revolution was driven forward by coal, and the steam railway engines which used to power across the British landscape were raised on a diet of coal mined in these islands. Heritage railways are already reporting difficulties in operating as a consequence of the bans on imports from Russia, and are even exploring using rapeseed sourced fuel. Steel producers also require coal. Earlier this year there was a temporary ban imposed locally on the export of coal from Indonesia. Will that be repeated now other sources are under pressure because of the conflict?
A possible consequence of these supply shortages and market disruption is that avoidable shortcuts might be taken in the shipment of commodities leading to problems during carriage. The carriage of coal has a number of associated hazards – self-heating, gas evolution, fires, liquefaction. Similarly, carriage of vegetable oils can give rise to substantial claims – contamination, admixture, overheating and oxidation, water damage and increase in free fatty acids.
Not the Tai Prize
It has recently been in the news in shipping circles that permission has not been granted for the TAI PRIZE dispute to be taken to the Supreme Court, and so the Court of Appeal judgement is the final word on the matter.
I wasn’t involved in the TAI PRIZE and I am not aware of the finer details of the case and what facts the arbitrator and judges had at their disposal. So, I’m not going to write about the TAI PRIZE, or the legal issues it deals with, but I am going to talk about soya beans and “apparent good order and condition”.
I have been inspecting cargoes of soya beans for more years than I’d like to say. It seems to be a commodity which causes a great deal of trouble worldwide. In recent years, the focus of many claims has been the trade from Brazil to China, but there have been plenty of cases involving soya beans of different origin and in different discharge ports.
As with agricultural materials generally, there is a tendency for soya beans to deteriorate microbiologically when overmoist. Unlike many other commodities, soya beans have a relatively large oil content. When vegetable oils oxidise, the chemical reaction releases heat. That means that soya beans can self-heat to rather higher temperatures than maize or rice. At elevated temperatures, further chemical reactions between proteins and sugars which result in the beans discolouring through a variety of shades of beige to brown and eventually black. These are known as Maillard reactions (nothing to do with ducks!) and they are also the reactions which take place when food is cooked.

People doing my sort of work tend to give presentations exhibiting lots of dramatic photographs like the above. They make good slides in powerpoint presentations! But these are photographs of holds in which there has been a major problem with soya beans. They aren’t representative of normal shipments.
Incidentally, the phrase “burned” is often bandied about to describe beans which are heavily darkened or even blackened. To actually set soya beans on fire requires quite a bit of effort, but it can be done. They then will burn, slowly. Heavily darkened beans where the darkness has arisen by means of elevated temperatures created by self-heating, and the Maillard reactions which result, are not burned.
And so to the “apparent good order and condition” point. This term has a legal meaning which hangs on the word “apparent” – it relates to what can be seen by normal inspections by a ship’s Master and the crew during loading.
The most recent grain case I have handled had just under 45000MT of grain loaded in 49 hours. That is on average just over 900MT an hour. A single soya bean weighs about 0.15g. So in each hour, some 6×109 beans are loaded. In American terms that is six billion; in British terms six thousand million beans.
Add to that the fact that the crew member in question might be standing by the hatch looking in. The nearest cargo is several metres away. There will be a cloud of dust hanging over the hold for much of the time, and there won’t be much chance of seeing through that cloud to notice anything much about the beans.
The first thing which definitely does not come under “apparent good order” is moisture content. This is a hugely important parameter as it plays a significant role in determining whether cargo will carry without deterioration. It isn’t a visible one, except in very extreme cases.
Soya beans which appear visibly wet would have to have a moisture content in excess of 30-40% and would be visibly saturated. However, the difference in moisture levels which give rise to problems in soya bean cargoes on ships are differences between 12%, 13%, 14% and so on. These all look identical. Very often I hear mouldy or otherwise damaged grain and soya bean cargoes referred to as being “wet”. They usually are not actually wet.
So, except in extreme cases, you can’t see problematic moisture contents. Sometimes we see recommendations for having a surveyor present to take small samples and measure moisture levels. There are portable moisture meters which can be used to get a rapid result on a ship. Obtaining a sample and using the meter might take 5 minutes if the surveyor is efficient. In that time in the example above some 75MT of cargo (500 million beans) may have been loaded. As the moisture content doesn’t have any impact on “apparent good order and condition”, what do you then do with that information? If the figures are such that an expert would tell you there is a strong chance of deterioration on a given voyage, what then do you do?
The next thing which isn’t “apparent good order” is the cargo temperature. You cannot see it. Is it a good idea for temperatures to be taken during loading? Wholeheartedly yes. There are lots of reasons why knowing the cargo temperature at loading can be useful. Soya beans which are significantly warmer than ambient temperature may represent parts of a cargo which have already started to deteriorate before shipment. That is certainly something you would want to know about, and it is likely that such cargo would also have elevated levels of mouldy or discoloured beans, and in all likelihood, an unpleasant odour.
Choosing the right thermometer for a given situation is quite important. Spear probes may be necessary for some situations and these can take time to use properly. For taking rapid and plentiful readings during loading of a cargo of soya beans, an inexpensive infra-red remote thermometer gun is ideal. These only report the temperature of the nearest surface in front of the gun, but that’s fine for such situations. Accuracy down to a fraction of a degree isn’t important but a real-time indication of whether warm cargo is being loaded can be.

The next biggie which is not part of “apparent good order” is cargo quality. With soya beans, there are a number of parameters. Foreign material (you can see seed pods and other foreign material in the photograph above) is one. Dust and other foreign material is often airborne during loading and it settles into a layer on top of the cargo during a break in loading. Those layers can often be seen in and amongst the cargo when it has heated – see the top photograph earlier in this article. Sales contracts have allowances for foreign material, and also other parameters such as split beans, heat damaged beans, and so on. To measure the levels of these it is necessary to take representative samples and have them analysed. This is well beyond the scope of what would be considered part of the duties of the crew during a loading, and quality matters are not relevant considerations for “apparent good order and condition”, even though the individual items of foreign material and the split beans etc are visible to the unaided eye.
So, having discussed a few categories of issue which are not “apparent good order and condition” items, and having said that quality parameters are not, what about a part of the cargo where the beans are discoloured, or mouldy, etc? This is where it gets more complicated. An example of an assembly consisting exclusively of darkened beans, or where such beans are in a majority, is in the top photograph above and the two below.


These photographs were taken during a cargo discharge, but if a significant quantity of such beans was loaded into one part of a ship’s hold, and then that inspected, it may well be considered not to be in “apparent good order and condition”. That is because a parcel of discoloured beans does not look like a normal parcel of soya beans.
What’s the likelihood of something like that being spotted? Answering that is much more difficult. The rapid rates of loading typical at grain terminals and the dust generated make it very difficult for even gross problems such as these to be detected. It would be very easy for even a large parcel of discoloured beans to be loaded and covered with other cargo without even the most attentive crew member noticing the problem.
Experts analysing evidence from a discharge may make the judgement that the cargo deteriorated because of its inherent condition (so-called inherent vice). Deciding that a certain parcel of cargo was already damaged at the time of loading is very much harder to do. Sometimes the evidence does suggest this. It is very much more difficult again to determine whether that could or should have been detected at loading.